Correction of intermittent hypoxia reduces inflammation in obese subjects with obstructive sleep apnea
- PMID: 28878129
- PMCID: PMC5621882
- DOI: 10.1172/jci.insight.94379
Correction of intermittent hypoxia reduces inflammation in obese subjects with obstructive sleep apnea
Abstract
Background: In obese subjects with obstructive sleep apnea (OSA), chronic intermittent hypoxia (CIH) may be linked to systemic and adipose tissue inflammation.
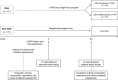
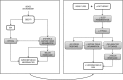
Methods: We obtained abdominal subcutaneous adipose tissue biopsies from OSA and non-OSA obese (BMI > 35) subjects at baseline and after 24 weeks (T1) of weight-loss intervention plus continuous positive airway pressure (c-PAP) or weight-loss intervention alone, respectively. OSA subjects were grouped according to good (therapeutic) or poor (subtherapeutic) adherence to c-PAP.
Results: At baseline, anthropometric and metabolic parameters, serum cytokines, and adipose tissue mRNA levels of obesity-associated chemokines and inflammatory markers were not different in OSA and non-OSA subjects. At T1, body weight was significantly reduced in all groups. Serum concentrations of IL-2, IL-4, IL-6, MCP-1, PDGFβ, and VEGFα were reduced by therapeutic c-PAP in OSA subjects and remained unaltered in non-OSA and subtherapeutic c-PAP groups. Similarly, adipose tissue mRNA levels of macrophage-specific (CD68, CD36) and ER stress (ATF4, CHOP, ERO-1) gene markers, as well as of IL-6, PDGFβ, and VEGFα, were decreased only in the therapeutic c-PAP group.
Conclusion: CIH does not represent an additional factor increasing systemic and adipose tissue inflammation in morbid obesity. However, in subjects with OSA, an effective c-PAP therapy improves systemic and obesity-associated inflammatory markers.
Funding: Ministero dell'Università e della Ricerca and Progetti di Rilevante Interesse Nazionale.
Keywords: Adipose tissue; Cytokines; Metabolism; Pulmonology; hypoxia.
Conflict of interest statement
Figures


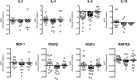


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

