Prevention of breast cancer skeletal metastases with parathyroid hormone
- PMID: 28878134
- PMCID: PMC5621896
- DOI: 10.1172/jci.insight.90874
Prevention of breast cancer skeletal metastases with parathyroid hormone
Abstract
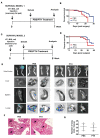
Advanced breast cancer is frequently associated with skeletal metastases and accelerated bone loss. Recombinant parathyroid hormone [teriparatide, PTH(1-34)] is the first anabolic agent approved in the US for treatment of osteoporosis. While signaling through the PTH receptor in the osteoblast lineage regulates bone marrow hematopoietic niches, the effects of anabolic PTH on the skeletal metastatic niche are unknown. Here, we demonstrate, using orthotopic and intratibial models of 4T1 murine and MDA-MB-231 human breast cancer tumors, that anabolic PTH decreases both tumor engraftment and the incidence of spontaneous skeletal metastasis in mice. Microcomputed tomography and histomorphometric analyses revealed that PTH increases bone volume and reduces tumor engraftment and volume. Transwell migration assays with murine and human breast cancer cells revealed that PTH alters the gene expression profile of the metastatic niche, in particular VCAM-1, to inhibit recruitment of cancer cells. While PTH did not affect growth or migration of the primary tumor, it elicited several changes in the tumor gene expression profile resulting in a less metastatic phenotype. In conclusion, PTH treatment in mice alters the bone microenvironment, resulting in decreased cancer cell engraftment, reduced incidence of metastases, preservation of bone microarchitecture and prolonged survival.
Keywords: Bone Biology; Bone disease; Breast cancer; G-protein coupled receptors; Oncology.
Conflict of interest statement
Figures








References
-
- Coleman RE. Clinical features of metastatic bone disease and risk of skeletal morbidity. Clin Cancer Res. 2006;12(20 Pt 2):6243s–6249s. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

