HSP90 inhibition enhances cancer immunotherapy by upregulating interferon response genes
- PMID: 28878208
- PMCID: PMC5587668
- DOI: 10.1038/s41467-017-00449-z
HSP90 inhibition enhances cancer immunotherapy by upregulating interferon response genes
Abstract
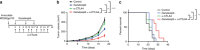
T-cell-based immunotherapies are promising treatments for cancer patients. Although durable responses can be achieved in some patients, many patients fail to respond to these therapies, underscoring the need for improvement with combination therapies. From a screen of 850 bioactive compounds, we identify HSP90 inhibitors as candidates for combination with immunotherapy. We show that inhibition of HSP90 with ganetespib enhances T-cell-mediated killing of patient-derived human melanoma cells by their autologous T cells in vitro and potentiates responses to anti-CTLA4 and anti-PD1 therapy in vivo. Mechanistic studies reveal that HSP90 inhibition results in upregulation of interferon response genes, which are essential for the enhanced killing of ganetespib treated melanoma cells by T cells. Taken together, these findings provide evidence that HSP90 inhibition can potentiate T-cell-mediated anti-tumor immune responses, and rationale to explore the combination of immunotherapy and HSP90 inhibitors.Many patients fail to respond to T cell based immunotherapies. Here, the authors, through a high-throughput screening, identify HSP90 inhibitors as a class of preferred drugs for treatment combination with immunotherapy.
Conflict of interest statement
The authors declare no competing financial interests.
Figures



References
-
- Peng W, et al. Loss of PTEN promotes resistance to T cell-mediated immunotherapy. Cancer Discov. 2016;6:202–216. doi: 10.1158/2159-8290.CD-15-0283. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

