PaxA, but not PaxC, is required for cnidocyte development in the sea anemone Nematostella vectensis
- PMID: 28878874
- PMCID: PMC5584322
- DOI: 10.1186/s13227-017-0077-7
PaxA, but not PaxC, is required for cnidocyte development in the sea anemone Nematostella vectensis
Abstract
Background: Pax genes are a family of conserved transcription factors that regulate many aspects of developmental morphogenesis, notably the development of ectodermal sensory structures including eyes. Nematostella vectensis, the starlet sea anemone, has numerous Pax orthologs, many of which are expressed early during embryogenesis. The function of Pax genes in this eyeless cnidarian is unknown.
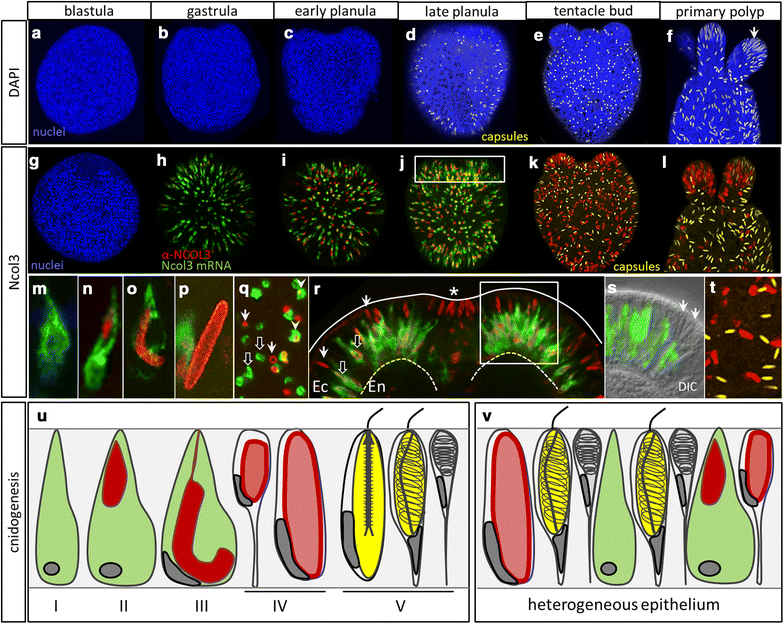
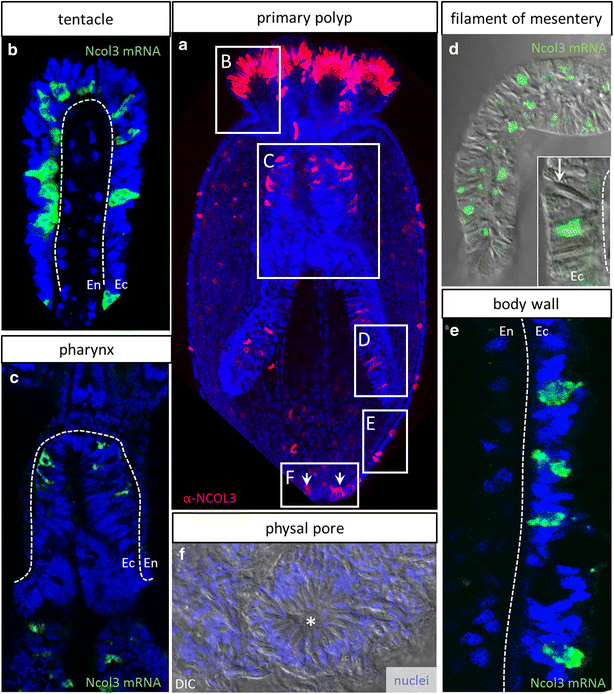
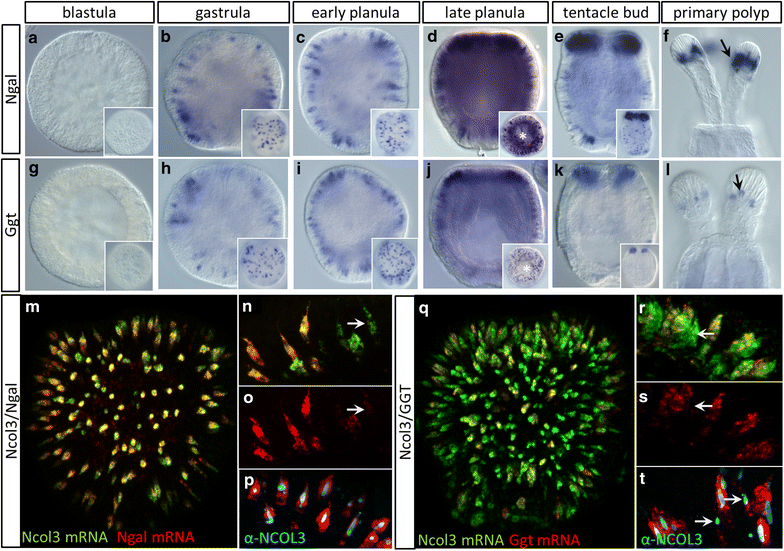
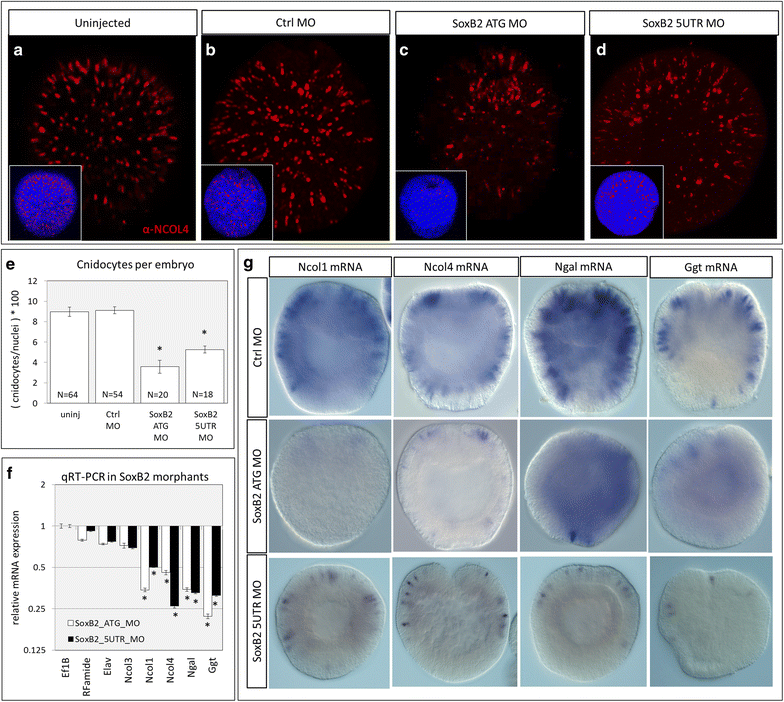
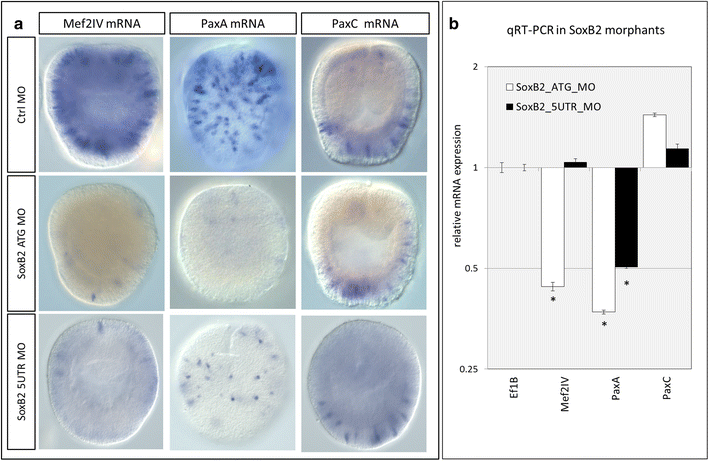
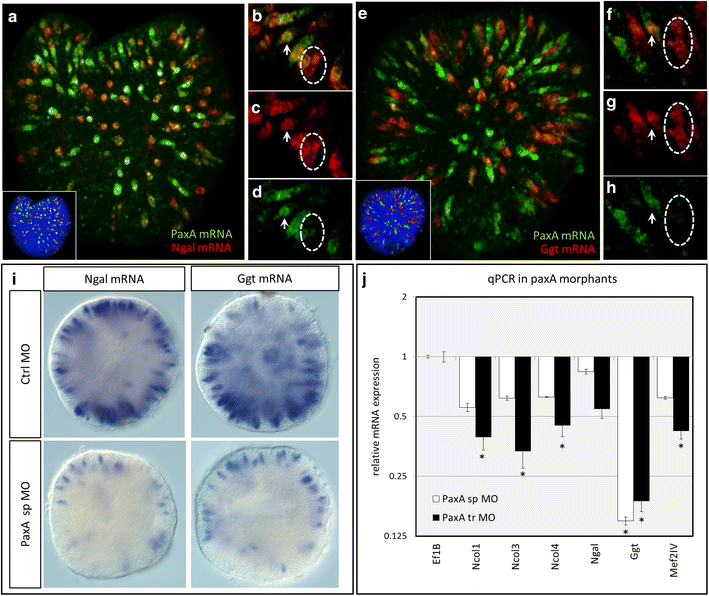
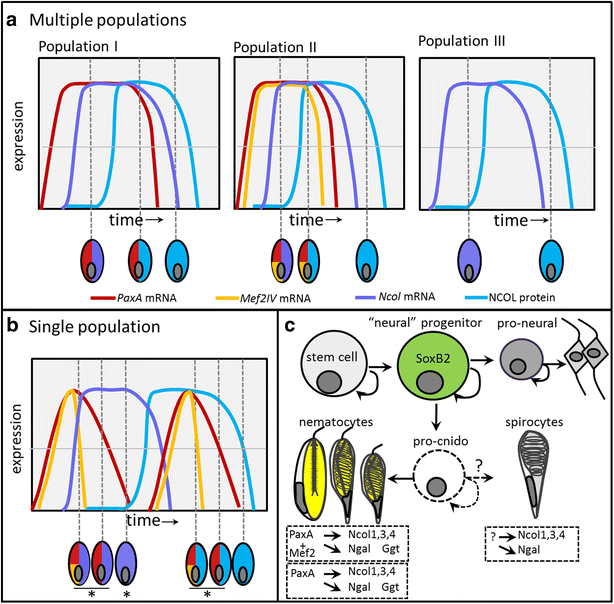
Results: Here, we show that PaxA, but not PaxC, plays a critical role in the development of cnidocytes in N. vectensis. Knockdown of PaxA results in a loss of developing cnidocytes and downregulation of numerous cnidocyte-specific genes, including a variant of the transcription factor Mef2. We also demonstrate that the co-expression of Mef2 in a subset of the PaxA-expressing cells is associated with the development with a second lineage of cnidocytes and show that knockdown of the neural progenitor gene SoxB2 results in downregulation of expression of PaxA, Mef2, and several cnidocyte-specific genes. Because PaxA is not co-expressed with SoxB2 at any time during cnidocyte development, we propose a simple model for cnidogenesis whereby a SoxB2-expressing progenitor cell population undergoes division to give rise to PaxA-expressing cnidocytes, some of which also express Mef2.
Discussion: The role of PaxA in cnidocyte development among hydrozoans has not been studied, but the conserved role of SoxB2 in regulating the fate of a progenitor cell that gives rise to neurons and cnidocytes in Nematostella and Hydractinia echinata suggests that this SoxB2/PaxA pathway may well be conserved across cnidarians.
Keywords: Cell differentiation; Evolution; Gene regulatory network; Mef2; Nematocyte; Novelty; PaxA; PaxC; SoxB2.
Figures




References
-
- Kass-Simon G, Scappaticci JAA. The behavioral and developmental physiology of nematocysts. Can J Zool. 2002;80:1772–1794. doi: 10.1139/z02-135. - DOI
LinkOut - more resources
Full Text Sources
Other Literature Sources

