Implementation of the World Health Organization Regional Office for Africa Stepwise Laboratory Quality Improvement Process Towards Accreditation
- PMID: 28879103
- PMCID: PMC5436392
- DOI: 10.4102/ajlm.v5i1.280
Implementation of the World Health Organization Regional Office for Africa Stepwise Laboratory Quality Improvement Process Towards Accreditation
Abstract
Background: The increase in disease burden has continued to weigh upon health systems in Africa. The role of the laboratory has become increasingly critical in the improvement of health for diagnosis, management and treatment of diseases. In response, the World Health Organization Regional Office for Africa (WHO AFRO) and its partners created the WHO AFRO Stepwise Laboratory (Quality) Improvement Process Towards Accreditation (SLIPTA) program.
Slipta implementation process: WHO AFRO defined a governance structure with roles and responsibilities for six main stakeholders. Laboratories were evaluated by auditors trained and certified by the African Society for Laboratory Medicine. Laboratory performance was measured using the WHO AFRO SLIPTA scoring checklist and recognition certificates rated with 1-5 stars were issued.
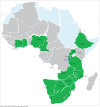
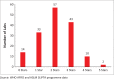
Preliminary results: By March 2015, 27 of the 47 (57%) WHO AFRO member states had appointed a SLIPTA focal point and 14 Ministers of Health had endorsed SLIPTA as the desired programme for continuous quality improvement. Ninety-eight auditors from 17 African countries, competent in the Portuguese (3), French (12) and English (83) languages, were trained and certified. The mean score for the 159 laboratories audited between May 2013 and March 2015 was 69% (median 70%; SD 11.5; interquartile range 62-77). Of these audited laboratories, 70% achieved 55% compliance or higher (2 or more stars) and 1% scored at least 95% (5 stars). The lowest scoring sections of the WHO AFRO SLIPTA checklist were sections 6 (Internal Audit) and 10 (Corrective Action), which both had mean scores below 50%.
Conclusion: The WHO AFRO SLIPTA is a process that countries with limited resources can adopt for effective implementation of quality management systems. Political commitment, ownership and investment in continuous quality improvement are integral components of the process.
Conflict of interest statement
The authors declare that they have no financial or personal relationship(s) that may have inappropriately influenced them in writing this article.
Figures
References
-
- Boutayeb A. The impact of infectious diseases on the development of Africa In: Preedy VR, Watson RR, editors. Handbook of disease burdens and quality of life measures. New York: Springer Science+Business Media, LLC., 2010; p. 1171–1188.
-
- Nkengasong JN. Strengthening laboratory services and systems in resource-poor countries. Am J Clin Pathol. 2009;131(6):774 http://dx.doi.org/10.1309/AJCP8GYX8KTKDATZ - DOI - PubMed
-
- Burgess DCH, Wasserman J, Dahl CA. Global health diagnostics. Nature. 2006;444(Suppl 1):1–2. http://dx.doi.org/10.1038/nature05440 - DOI - PubMed
-
- Peter TF, Rotz PD, Blair DH, et al. Impact of laboratory accreditation on patient care and the health system. Am J Clin Pathol. 2010;134(4):550–555. http://dx.doi.org/10.1309/AJCPH1SKQ1HNWGHF - DOI - PubMed
-
- Fu E, Yager P, Floriano PN, et al. Perspective on diagnostics for global health. IEEE Pulse. 2011;2(6):40–50. http://dx.doi.org/10.1109/MPUL.2011.942766 - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources