Child and adolescent self-report symptom measurement in pediatric oncology research: a systematic literature review
- PMID: 28879501
- PMCID: PMC5823735
- DOI: 10.1007/s11136-017-1692-4
Child and adolescent self-report symptom measurement in pediatric oncology research: a systematic literature review
Abstract
Objective: Previous work in pediatric oncology has found that clinicians and parents tend to under-report the frequency and severity of treatment-related symptoms compared to child self-report. As such, there is a need to identify high-quality self-report instruments to be used in pediatric oncology research studies. This study's objective was to conduct a systematic literature review of existing English language instruments used to measure self-reported symptoms in children and adolescents undergoing cancer treatment.
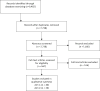
Methods: A comprehensive literature search was conducted in MEDLINE/PubMed, EMBASE, CINAHL, and PsycINFO to identify relevant articles published through November 10, 2016. Using pre-specified inclusion/exclusion criteria, six trained reviewers carefully screened abstracts and full-text articles for eligibility.
Results: There were 7738 non-duplicate articles identified in the literature search. Forty articles met our eligibility criteria, and within these articles, there were 38 self-report English symptom instruments. Most studies evaluated only cross-sectional psychometric properties, such as reliability or validity. Ten studies assessed an instrument's responsiveness or ability to detect changes in symptoms over time. Eight instruments met our criteria for use in future longitudinal pediatric oncology studies.
Conclusions: This systematic review aids pediatric oncology researchers in identifying and selecting appropriate symptom measures with strong psychometric evidence for their studies. Enhancing the child's voice in pediatric oncology research studies allows us to better understand the impact of cancer and its treatment on the lives of children.
Keywords: Adverse event self-report; Pediatric oncology; Self-report instruments.
Conflict of interest statement
Figures
Similar articles
-
Eliciting adverse effects data from participants in clinical trials.Cochrane Database Syst Rev. 2018 Jan 16;1(1):MR000039. doi: 10.1002/14651858.MR000039.pub2. Cochrane Database Syst Rev. 2018. PMID: 29372930 Free PMC article.
-
The measurement and monitoring of surgical adverse events.Health Technol Assess. 2001;5(22):1-194. doi: 10.3310/hta5220. Health Technol Assess. 2001. PMID: 11532239
-
A systematic review of tools designed for teacher proxy-report of children's physical literacy or constituting elements.Int J Behav Nutr Phys Act. 2021 Oct 8;18(1):131. doi: 10.1186/s12966-021-01162-3. Int J Behav Nutr Phys Act. 2021. PMID: 34620185 Free PMC article.
-
Home treatment for mental health problems: a systematic review.Health Technol Assess. 2001;5(15):1-139. doi: 10.3310/hta5150. Health Technol Assess. 2001. PMID: 11532236
-
Music interventions for improving psychological and physical outcomes in people with cancer.Cochrane Database Syst Rev. 2021 Oct 12;10(10):CD006911. doi: 10.1002/14651858.CD006911.pub4. Cochrane Database Syst Rev. 2021. PMID: 34637527 Free PMC article.
Cited by
-
Consensus Recommendations From the Children's Oncology Group Nursing Discipline's State of the Science Symposium: Symptom Assessment During Childhood Cancer Treatment.J Pediatr Oncol Nurs. 2019 Jul/Aug;36(4):294-299. doi: 10.1177/1043454219854983. J Pediatr Oncol Nurs. 2019. PMID: 31307318 Free PMC article. Review.
-
Symptom screening in paediatrics tool for screening multiple symptoms in Brazilian patients with cancer: a cross-sectional validation study.BMJ Open. 2019 Aug 2;9(8):e028149. doi: 10.1136/bmjopen-2018-028149. BMJ Open. 2019. PMID: 31377698 Free PMC article.
-
Beyond the storm - subacute toxicities and late effects in children receiving CAR T cells.Nat Rev Clin Oncol. 2021 Jun;18(6):363-378. doi: 10.1038/s41571-020-00456-y. Epub 2021 Jan 25. Nat Rev Clin Oncol. 2021. PMID: 33495553 Free PMC article. Review.
-
Agreement Level of Inflammatory Bowel Disease Symptom Reports between Children and Their Parents.Pediatr Gastroenterol Hepatol Nutr. 2023 Mar;26(2):88-98. doi: 10.5223/pghn.2023.26.2.88. Epub 2023 Mar 7. Pediatr Gastroenterol Hepatol Nutr. 2023. PMID: 36950060 Free PMC article.
-
The Symptom Experience in Pediatric Cancer: Current Conceptualizations and Future Directions.Curr Oncol Rep. 2022 Apr;24(4):443-450. doi: 10.1007/s11912-022-01222-2. Epub 2022 Feb 12. Curr Oncol Rep. 2022. PMID: 35150393 Review.
References
-
- American Cancer Society. Cancer facts & figures 2016. Atlanta: American Cancer Society; 2016. p. 11.
-
- CureSearch Childhood Cancer Statistics. http://curesearch.org/Childhood-Cancer-Statistics.
-
- Sateren WB, Trimble EL, Abrams J, Brawley O, Breen N, Ford L, et al. How sociodemographics, presence of oncology specialists, and hospital cancer programs affect accrual to cancer treatment trials. Journal of Clinical Oncology. 2002;20(8):2109–2117. - PubMed
-
- Collins JJ, Byrnes ME, Dunkel IJ, Lapin J, Nadel T, Thaler HT, et al. The measurement of symptoms in children with cancer. Journal of Pain and Symptom Management. 2000;19(5):363–377. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous