Electronic and Structural Properties of ABO3: Role of the B-O Coulomb Repulsions for Ferroelectricity
- PMID: 28879987
- PMCID: PMC5448477
- DOI: 10.3390/ma4010260
Electronic and Structural Properties of ABO3: Role of the B-O Coulomb Repulsions for Ferroelectricity
Abstract
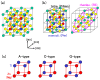
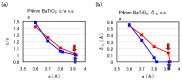
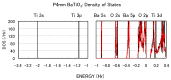
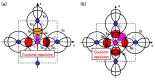
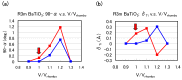
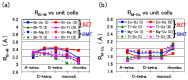
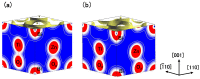
We have investigated the role of the Ti-O Coulomb repulsions in the appearance of the ferroelectric state in BaTiO3 as well as the role of the Zn-O Coulomb repulsions in BiZn0.5Ti0.5O3, using a first-principles calculation with optimized structures. In tetragonal BaTiO3, it is found that the Coulomb repulsions between Ti 3s and 3p states and O 2s and 2p states have an important role for the appearance of Ti ion displacement. In BiZn0.5Ti0.5O3, on the other hand, the stronger Zn-O Coulomb repulsions, which are due to the 3s, 3p, and 3d (d10) states of the Zn ion, have more important role than the Ti-O Coulomb repulsions for the appearance of the tetragonal structure. Our suggestion is consistent with the other ferroelectric perovskite oxides ABO3 in the appearance of tetragonal structures as well as rhombohedral structures.
Keywords: coulomb repulsion; electronic band structure; ferroelectrics; phase transitions.
Figures


Similar articles
-
Annealing-Dependent Morphotropic Phase Boundary in the BiMg0.5Ti0.5O3-BiZn0.5Ti0.5O3 Perovskite System.Materials (Basel). 2022 Oct 9;15(19):6998. doi: 10.3390/ma15196998. Materials (Basel). 2022. PMID: 36234350 Free PMC article.
-
Relationship among the Crystal Structure, Texture, and Macroscopic Properties of Tetragonal (Pb,La)(Zr,Ti)O3 Ferroelectrics Investigated by In Situ High-Energy Synchrotron Diffraction.Inorg Chem. 2020 Sep 21;59(18):13632-13638. doi: 10.1021/acs.inorgchem.0c02002. Epub 2020 Aug 30. Inorg Chem. 2020. PMID: 32862641
-
High-Pressure Synthesis, Crystal Structure, Chemical Bonding, and Ferroelectricity of LiNbO3-Type LiSbO3.Inorg Chem. 2018 Dec 17;57(24):15462-15473. doi: 10.1021/acs.inorgchem.8b02767. Epub 2018 Dec 3. Inorg Chem. 2018. PMID: 30507117
-
First-principles study for vacancy-induced magnetism in nonmagnetic ferroelectric BaTiO3.Phys Chem Chem Phys. 2009 Dec 14;11(46):10934-8. doi: 10.1039/b908058a. Epub 2009 Sep 22. Phys Chem Chem Phys. 2009. PMID: 19924328
-
Brillouin Scattering and First-Principles Studies of BaMO3 (M = Ti, Zr, and Cu) Perovskites.Materials (Basel). 2022 Sep 28;15(19):6747. doi: 10.3390/ma15196747. Materials (Basel). 2022. PMID: 36234088 Free PMC article. Review.
Cited by
-
Anisotropic Piezoelectric Properties of Porous (Ba0.85Ca0.15)(Zr0.1Ti0.9)O3 Ceramics with Oriented Pores through TBA-Based Freeze-Casting Method.Materials (Basel). 2022 May 27;15(11):3820. doi: 10.3390/ma15113820. Materials (Basel). 2022. PMID: 35683118 Free PMC article.
References
Publication types
LinkOut - more resources
Full Text Sources

