Sol-Gel Entrapped Levonorgestrel Antibodies: Activity and Structural Changes as a Function of Different Polymer Formats
- PMID: 28880001
- PMCID: PMC5448502
- DOI: 10.3390/ma4030469
Sol-Gel Entrapped Levonorgestrel Antibodies: Activity and Structural Changes as a Function of Different Polymer Formats
Abstract
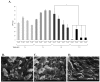
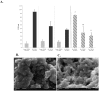
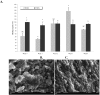
The paper describes development of a sol-gel based immunoaffinity method for the steroid hormone levonorgestrel (LNG) and the effects of changes in the sol-gel matrix format on the activity of the entrapped antibodies (Abs) and on matrix structure. The best sol-gel format for Ab entrapment was found to be a tetramethoxysilane (TMOS) based matrix at a TMOS:water ratio of 1:8, containing 10% polyethylene glycol (PEG) of MW 0.4 kDa. Addition of higher percentages of PEG or a higher MW PEG did not improve activity. No activity was obtained with a TMOS:water ratio of 1:12, most likely because of the very dense polymer that resulted from these polymerization conditions. Only minor differences in the non-specific binding were obtained with the various formats. TMOS was found to be more effective than tetrakis (2-hydroxyethyl)orthosilicate (THEOS) for entrapment of anti-levonorgestrel (LNG) Abs. However, aging the THEOS-based sol-gel for a few weeks at 4 °C stabilized the entrapped Abs and increased its binding capacity. Confocal fluorescent microscopy with fluorescein isothiocyanate (FITC) labeled immunoglobulines (IgGs) entrapped in the sol-gel matrix showed that the entrapped Abs were distributed homogenously within the gel. Scanning electron microscopy (SEM) images have shown the diverse structures of the various sol-gel formats and precursors.
Keywords: THEOS; TMOS; antibodies; immunoaffinity purification; levonorgestrel; polyethylene glycol; sol-gel.
Figures



References
-
- Avnir D., Braun S. Biochemical Aspects of Sol-Gel Science and Technology. Kluwer Academic Publishers; Boston, MA, USA: 1996.
-
- Kandimalla V.B., Tripathi V.S., Ju H.X. Immobilization of biomolecules in sol-gels: Biological and analytical applications. Crit. Rev. Anal. Chem. 2006;36:73–106. doi: 10.1080/10408340600713652. - DOI
-
- Pierre A.C. The sol-gel encapsulation of enzymes. Biocatal. Biotransform. 2004;22:145–170. doi: 10.1080/10242420412331283314. - DOI
-
- Braun S., Rappoport S., Zusman R., Avnir D., Ottolenghi M. Biochemically active sol-gel glasses—the trapping of enzymes. Mater. Lett. 1990;10:1–5. doi: 10.1016/0167-577X(90)90002-4. - DOI
LinkOut - more resources
Full Text Sources
Other Literature Sources

