PVR: Patch-to-Volume Reconstruction for Large Area Motion Correction of Fetal MRI
- PMID: 28880160
- PMCID: PMC6051489
- DOI: 10.1109/TMI.2017.2737081
PVR: Patch-to-Volume Reconstruction for Large Area Motion Correction of Fetal MRI
Abstract
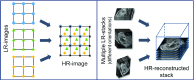
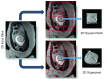
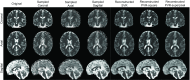
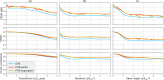
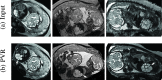
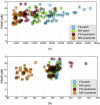
In this paper, we present a novel method for the correction of motion artifacts that are present in fetal magnetic resonance imaging (MRI) scans of the whole uterus. Contrary to current slice-to-volume registration (SVR) methods, requiring an inflexible anatomical enclosure of a single investigated organ, the proposed patch-to-volume reconstruction (PVR) approach is able to reconstruct a large field of view of non-rigidly deforming structures. It relaxes rigid motion assumptions by introducing a specific amount of redundant information that is exploited with parallelized patchwise optimization, super-resolution, and automatic outlier rejection. We further describe and provide an efficient parallel implementation of PVR allowing its execution within reasonable time on commercially available graphics processing units, enabling its use in the clinical practice. We evaluate PVR's computational overhead compared with standard methods and observe improved reconstruction accuracy in the presence of affine motion artifacts compared with conventional SVR in synthetic experiments. Furthermore, we have evaluated our method qualitatively and quantitatively on real fetal MRI data subject to maternal breathing and sudden fetal movements. We evaluate peak-signal-to-noise ratio, structural similarity index, and cross correlation with respect to the originally acquired data and provide a method for visual inspection of reconstruction uncertainty. We further evaluate the distance error for selected anatomical landmarks in the fetal head, as well as calculating the mean and maximum displacements resulting from automatic non-rigid registration to a motion-free ground truth image. These experiments demonstrate a successful application of PVR motion compensation to the whole fetal body, uterus, and placenta.
Figures








References
-
- Mansfield P. and Maudsley A. A., “Planar spin imaging by NMR,” J. Magn. Reson., vol. 27, no. 1, pp. 101–119, 1977.
-
- Ertl-Wagner B., Lienemann A., Strauss A., and Reiser M. F., “Fetal magnetic resonance imaging: Indications, technique, anatomical considerations and a review of fetal abnormalities,” Eur. Radiol., vol. 12, no. 8, pp. 1931–1940, 2002. - PubMed
-
- Huppert B., Brandt K., Ramin K., and King B., “Single-shot fast spin-echo MR imaging of the fetus: A pictorial essay,” Radiographics, vol. 19, no. 1, p. 10, 1999. - PubMed
-
- Garel C., “Imaging the fetus: When does MRI really help?” Pediatric Radiol., vol. 38, pp. 467–470, Jun. 2008. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

