Colorectal cancer at high risk of peritoneal metastases: long term outcomes of a pilot study on adjuvant laparoscopic HIPEC and future perspectives
- PMID: 28881641
- PMCID: PMC5584242
- DOI: 10.18632/oncotarget.17158
Colorectal cancer at high risk of peritoneal metastases: long term outcomes of a pilot study on adjuvant laparoscopic HIPEC and future perspectives
Abstract
Objective: Early detection of peritoneal metastases (PM) of colorectal cancer (CRC) is difficult and treatment options at a clinically overt stage are limited. Potentially, adjuvant laparoscopic hyperthermic intraperitoneal chemotherapy (HIPEC) is of value. The aim of this study was to present long term oncological outcomes of a pilot study on adjuvant HIPEC to reduce development of PMCRC, with systematic review of literature.
Methods: Long term oncological outcomes of ten patients who underwent laparoscopic HIPEC within eight weeks after resection of primary CRC in the pilot study were retrospectively collected. A systematic search of literature was performed on studies describing the use of HIPEC in patients with CRC at high risk of developing PM.
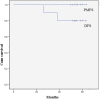
Results: The median follow-up was 54 months (range 49-63). All patients were alive at the last follow-up moment and none of them had developed PM. Two patients had developed pulmonary metastases. Systematic review revealed five small cohort studies, including two matched comparisons. Peritoneal recurrences were found in 0% to 9% after adjuvant HIPEC, which was 28% and 43% in the two control groups, respectively. Disease free and overall survival were significantly higher in favour of HIPEC.
Conclusion: Long term follow-up of ten patients included in a pilot study on adjuvant HIPEC revealed no peritoneal recurrences. This result is in line with other published pilot studies, a promising observation. However, the outcomes of the Dutch randomized COLOPEC trial and similar trials worldwide should be awaited for definitive conclusions on the effectiveness of adjuvant HIPEC.
Keywords: adjuvant HIPEC; colorectal cancer; peritoneal metastases.
Conflict of interest statement
CONFLICTS OF INTEREST The authors have no conflicts of interest to declare.
Figures
References
-
- Verwaal VJ, van Ruth S, de Bree E, van Sloothen GW, van Tinteren H, Boot H, Zoetmulder FA. Randomized trial of cytoreduction and hyperthermic intraperitoneal chemotherapy versus systemic chemotherapy and palliative surgery in patients with peritoneal carcinomatosis of colorectal cancer. J Clin Oncol. 2003;21:3737–3743. - PubMed
-
- Pelz JO, Chua TC, Esquivel J, Stojadinovic A, Doerfer J, Morris DL, Maeder U, Germer CT, Kerscher AG. Evaluation of best supportive care and systemic chemotherapy as treatment stratified according to the retrospective peritoneal surface disease severity score (PSDSS) for peritoneal carcinomatosis of colorectal origin. BMC Cancer. 2010;10:689. - PMC - PubMed
-
- Franko J, Shi Q, Goldman CD, Pockaj BA, Nelson GD, Goldberg RM, Pitot HC, Grothey A, Alberts SR, Sargent DJ. Treatment of colorectal peritoneal carcinomatosis with systemic chemotherapy: a pooled analysis of north central cancer treatment group phase III trials N9741 and N9841. J Clin Oncol. 2012;30:263–267. - PMC - PubMed
-
- Klaver YL, Lemmens VE, Creemers GJ, Rutten HJ, Nienhuijs SW, de Hingh IH. Population-based survival of patients with peritoneal carcinomatosis from colorectal origin in the era of increasing use of palliative chemotherapy. Ann Oncol. 2011;22:2250–2256. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources