The effect of intraperitoneal chemotherapy on early pain hyperalgesia in patients following elective laparoscopic transabdominal resection of rectal cancer
- PMID: 28881696
- PMCID: PMC5584297
- DOI: 10.18632/oncotarget.18417
The effect of intraperitoneal chemotherapy on early pain hyperalgesia in patients following elective laparoscopic transabdominal resection of rectal cancer
Abstract
Background: Chemotherapy has been associated with hyperalgesia. This prospective study was designed to investigate the effect of intraperitoneal chemotherapy with lobaplatin on post-operative pain intensity and sufentanil requirements after laparoscopic transabdominal resection of rectal cancer.
Methods: Eighty subjects (40 subjects treated with intraperitoneal chemotherapy and 40 subjects without chemotherapy treatment) scheduled for laparoscopic transabdominal resection of rectal cancer were included in this study. All subjects received standardized anesthetic and patient-controlled analgesia using sufentanil for 72 h post-surgery, as the only analgesics. Pain intensity scores, cumulative sufentanil requirements and side effects were recorded until 72 h post-surgery.
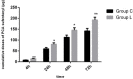
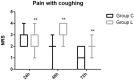
Results: Following intraperitoneal chemotherapy, patients had a significantly higher total post-operative sufentanil requirement (193 μg vs. 142 μg; P = 0.008), significantly higher verbal rating scale post-surgery pain intensity scores at rest and with coughing (P < 0.05), and a significantly worse functional activity score (P < 0.05) over 72 h, compared with those without intraperitoneal chemotherapy. There were no post-operative differences in the incidence of side-effects (post-operative nausea [P = 0.189], vomiting [P = 0.311], pruritus [P = 0.263], respiratory depression [P = 1.000], and dizziness [P = 0.712]) between the two groups.
Conclusion: Intraperitoneal chemotherapy is associated with significantly increased post-operative sufentanil requirements and pain intensity, suggesting chemotherapy-associated hyperalgesia.
Keywords: chemotherapy; colorectal cancer; hyperalgesia; lobaplatin; sufentanil.
Conflict of interest statement
CONFLICT OF INTEREST The authors declared that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
-
- Kautio AL, Haanpää M, Kautiainen H, Kalso E, Saarto T. Burden of chemotherapy-induced neuropathy-a cross-sectional study. Support Care Cancer. 2011;19:1991–6. - PubMed
-
- Smith EM, Cohen JA, Pett MA, Beck SL. The reliability and validity of a modified total neuropathy score-reduced and neuropathic pain severity items when used to measure chemotherapy-induced peripheral neuropathy in patients receiving taxanes and platinums. Cancer Nurs. 2010;33:173–83. - PubMed
-
- Quasthoff S, Hartung HP. Chemotherapy-induced peripheral neuropathy. J Neurol. 2002;249:9–17. - PubMed
-
- Smith EM, Bakitas MA, Homel P, Piehl M, Kingman L, Fadul CE, Bookbinder M. Preliminary assessment of a neuropathic pain treatment and referral algorithm for patients with cancer. J Pain Symptom Manage. 2011;42:822–38. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

