Value of quantitative magnetic resonance imaging T1-relaxometry in predicting contrast-enhancement in glioblastoma patients
- PMID: 28881830
- PMCID: PMC5581129
- DOI: 10.18632/oncotarget.18612
Value of quantitative magnetic resonance imaging T1-relaxometry in predicting contrast-enhancement in glioblastoma patients
Abstract
Summarizing the importance of the study: The repetitive usage of gadolinium-based contrast agents (GBCA) is critical for magnetic resonance imaging (MRI) evaluation of tumor burden in glioblastoma patients. It is also a crucial tool for determination of radiographical response to treatment. GBCA injection, however, comes with a 2.4% rate of adverse events including life-threatening conditions such as nephrogenic systemic fibrosis (NSF). Moreover, GBCA have been shown to be deposited in brain tissue of patients even with an intact blood-brain barrier (BBB). The present study explores quantitative T1 relaxometry as an alternative non-invasive imaging technique detection of tumor burden and determination of radiographical response. This technique exploits specific properties of brain tissue with impaired BBB. With a sensitivity and specificity as high as 86% and 80%, respectively, quantitative T1-relaxometry allows for detecting contrast-enhancing areas without the use of GBCA. This method could make it unnecessary to subject patients to the risk of adverse events associated with the use of GBCA. Nonetheless, a large-scale analysis is needed to confirm our findings.
Background: Gadolinium-based contrast agents (GBCA) are crucial for magnetic resonance imaging (MRI)-based evaluation of tumor burden in glioblastoma (GBM). Serious adverse events of GBCA, even though uncommon, and gadolinium deposition in brain tissue could be avoided by novel imaging techniques not requiring GBCA. Altered tissue composition in areas with impaired blood-brain-barrier also alters the quantified T1 relaxation time (qT1), so that qT1 analysis could replace GBCA-based MRI for the analysis of tumor burden and response.
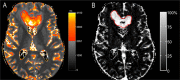
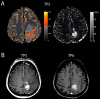
Methods: As a part of a prospective pilot MRI-relaxometry trial, patients with newly-diagnosed GBM who relapsed under standard radiochemotherapy were selected for this study. At recurrence, subtraction of qT1 maps pre and post-GBCA application (ΔqT1 maps) was used to determine areas of contrast-enhancement. With the contrast-enhancement on ΔqT1 maps as reference, ROC analysis served to detect an optimal qT1 cut-off on qT1 maps prior to GBCA to distinguish between contrast-enhancing tissue and its surroundings.
Results: Ten patients were included. A qT1 value >2051ms predicted contrast-enhancing tumor tissue with a sensitivity of 86% and specificity of 80% (AUC, 0.92; p<0.0001). Interestingly, qT1 prolongation >2051 ms that did not overlap with contrast-enhancing area transformed into contrast-enhancement later on (n=4).
Conclusion: T1-relaxometry may be a useful technique to assess tissue properties equivalent to contrast-enhancement without the need for GBCA application. It may also provide information on sites with future tumor progression. Nonetheless, large-scale studies are needed to confirm these findings.
Keywords: BBB damage; T1-mapping; T1-relaxometry; glioblastoma; quantitative MRI.
Conflict of interest statement
CONFLICTS OF INTEREST There is no conflicts of interest to declare.
Figures


References
-
- Macdonald DR, Cascino TL, Schold SC, Jr, Cairncross JG. Response criteria for phase II studies of supratentorial malignant glioma. J Clin Oncol. 1990;8:1277–1280. - PubMed
-
- Wen PY, Macdonald DR, Reardon DA, Cloughesy TF, Sorensen AG, Galanis E, Degroot J, Wick W, Gilbert MR, Lassman AB, Tsien C, Mikkelsen T, Wong ET, et al. Updated response assessment criteria for high-grade gliomas: response assessment in neuro-oncology working group. J Clin Oncol. 2010;28:1963–1972. - PubMed
-
- Stupp R, Mason WP, van den Bent MJ, Weller M, Fisher B, Taphoorn MJ, Belanger K, Brandes AA, Marosi C, Bogdahn U, Curschmann J, Janzer RC, Ludwin SK, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352:987–996. - PubMed
-
- Kanal E. Gadolinium based contrast agents (GBCA): Safety overview after 3 decades of clinical experience. Magn Reson Imaging. 2016;34:1341–1345. - PubMed
-
- McDonald RJ, McDonald JS, Kallmes DF, Jentoft ME, Murray DL, Thielen KR, Williamson EE, Eckel LJ. Intracranial gadolinium deposition after contrast-enhanced MR imaging. Radiology. 2015;275:772–782. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

