Cost-effectiveness analysis of quadrivalent seasonal influenza vaccines in England
- PMID: 28882149
- PMCID: PMC5590113
- DOI: 10.1186/s12916-017-0932-3
Cost-effectiveness analysis of quadrivalent seasonal influenza vaccines in England
Abstract
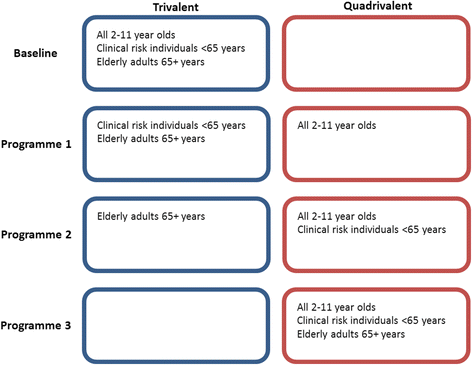
Background: As part of the national seasonal influenza vaccination programme in England and Wales, children receive a quadrivalent vaccine offering protection against two influenza A strains and two influenza B strains. Healthy children receive a quadrivalent live attenuated influenza vaccine (QLAIV), whilst children with contraindications receive the quadrivalent inactivated influenza vaccine (QIIV). Individuals aged younger than 65 years in the clinical risk populations and elderly individuals aged 65+ years receive either a trivalent inactivated influenza vaccine (TIIV) offering protection from two A strains and one B strain or the QIIV at the choice of their general practitioner. The cost-effectiveness of quadrivalent vaccine programmes is an open question. The original analysis that supported the paediatric programme only considered a trivalent live attenuated vaccine (LAIV). The cost-effectiveness of the QIIV to other patients has not been established. We sought to estimate the cost-effectiveness of these programmes, establishing a maximum incremental total cost per dose of quadrivalent vaccines over trivalent vaccines.
Methods: We used the same mathematical model as the analysis that recommended the introduction of the paediatric influenza vaccination programme. The incremental cost of the quadrivalent vaccine is the additional cost over that of the existing trivalent vaccine currently in use.
Results: Introducing quadrivalent vaccines can be cost-effective for all targeted groups. However, the cost-effectiveness of the programme is dependent on the choice of target cohort and the cost of the vaccines: the paediatric programme is cost-effective with an increased cost of £6.36 per dose, though an extension to clinical risk individuals younger than 65 years old and further to all elderly individuals means the maximum incremental cost is £1.84 and £0.20 per dose respectively.
Conclusions: Quadrivalent influenza vaccines will bring substantial health benefits, as they are cost-effective in particular target groups.
Keywords: Cost-effectiveness; Influenza; LAIV; QALY; Quadrivalent vaccines; Vaccination.
Conflict of interest statement
Competing interests
The authors declare that they have no competing interests.
Consent for publication
Not applicable.
Ethics approval and consent to participate
Not applicable.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures
References
-
- Public Health England. The national flu immunisation programme 2016/17. 2016. https://www.gov.uk/government/uploads/system/uploads/attachment_data/fil.... Accessed 13 Oct 2016.
-
- Pebody R, Warburton F, Andrews N, Ellis J, Von Wissmann B, Robertson C, et al. Effectiveness of seasonal influenza vaccine in preventing laboratory-confirmed influenza in primary care in the United Kingdom: 2014/15 end of season results. Eurosurveillance. 2015;20. doi:10.2807/1560-7917.ES.2015.20.36.30013 - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials