Estrogen attenuates the spondyloarthritis manifestations of the SKG arthritis model
- PMID: 28882159
- PMCID: PMC5590166
- DOI: 10.1186/s13075-017-1407-9
Estrogen attenuates the spondyloarthritis manifestations of the SKG arthritis model
Abstract
Background: Ankylosing spondylitis (AS) is a male-predominant disease, and radiographic evidence of damage is also more severe in males. Estrogen modulates immune-related processes such as T cell differentiation and cytokine production. This study aimed to evaluate the effect of estrogen on the disease activity of spondyloarthritis (SpA).
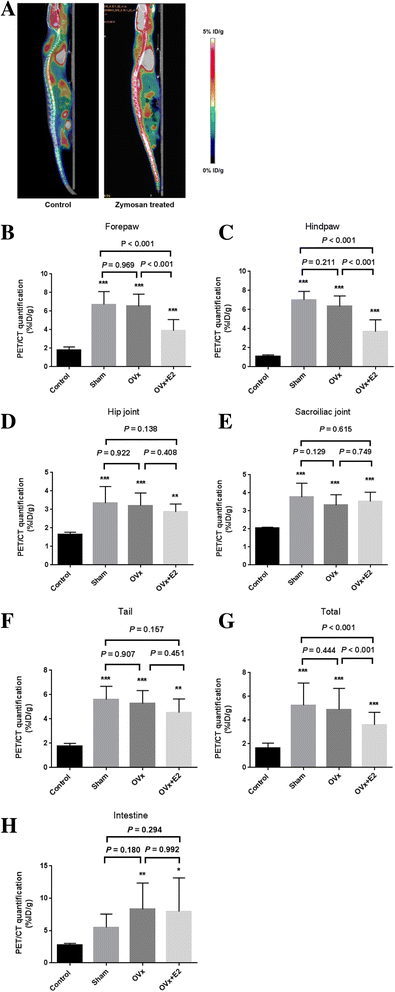
Methods: The effects of estrogen on the development of arthritis were evaluated by performing ovariectomy and 17β-estradiol (E2) pellet implantation in zymosan-treated SKG mice. Clinical arthritis scores were measured, and 18F-fluorodeoxyglucose (18F-FDG) small-animal positron emission tomography/computed tomography performed to quantify joint inflammation. The expression of inflammatory cytokines in joint tissue was measured.
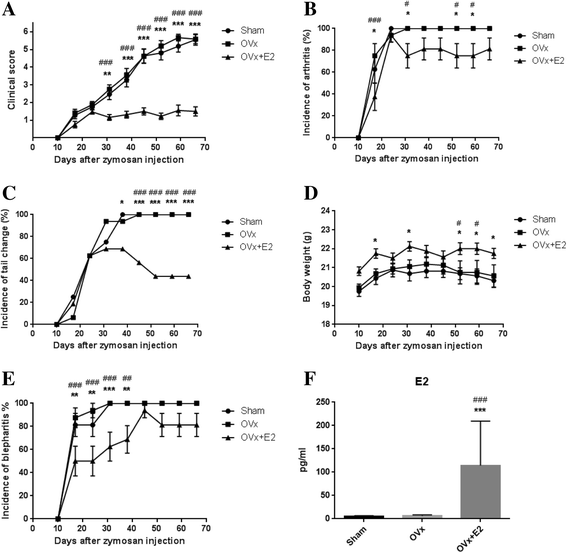
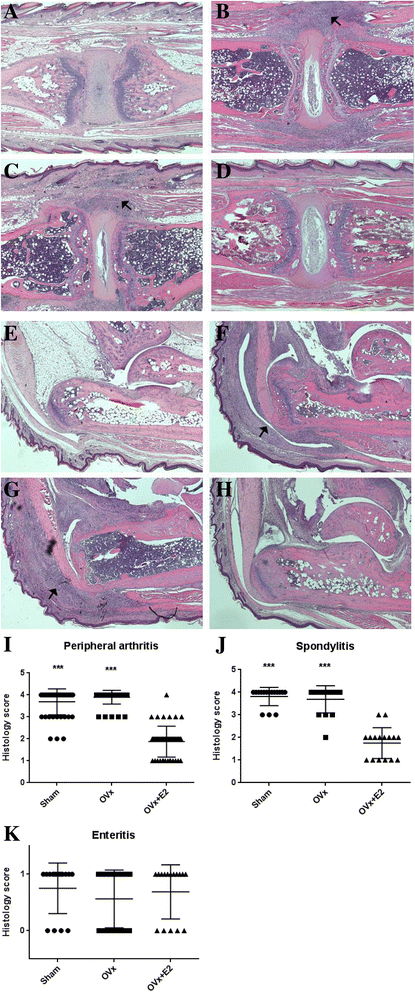
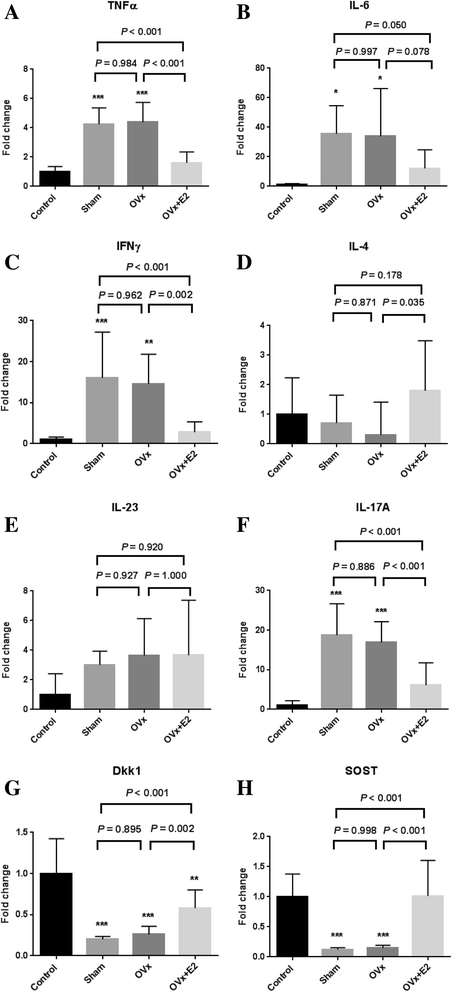
Results: E2-treated mice showed remarkable suppression of arthritis clinically and little infiltration of inflammatory cells in the Achilles tendon and intervertebral disc. 18F-FDG uptake was significantly lower in E2-treated mice than in sham-operated (sham) and ovariectomized mice. Expression of TNF, interferon-γ, and IL-17A was significantly reduced in E2-treated mice, whereas expression of sclerostin and Dickkopf-1 was increased in E2-treated mice compared with sham and ovariectomized mice.
Conclusions: Estrogen suppressed arthritis development in SKG mice, a model of SpA. Results of this study suggest that estrogen has an anti-inflammatory effect on the spondyloarthritis manifestations of the SKG arthritis model.
Keywords: Estrogen; Mice; Spondyloarthritis.
Conflict of interest statement
Ethics approval
All animal studies were approved by the Institutional Animal Care and Use Committee at Samsung Medical Center.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures
References
-
- Rudwaleit M, van der Heijde D, Landewe R, Listing J, Akkoc N, Brandt J, et al. The development of Assessment of SpondyloArthritis international Society classification criteria for axial spondyloarthritis (part II): validation and final selection. Ann Rheum Dis. 2009;68:777–83. doi: 10.1136/ard.2009.108233. - DOI - PubMed
-
- Oostveen J, Prevo R, den Boer J, van de Laar M. Early detection of sacroiliitis on magnetic resonance imaging and subsequent development of sacroiliitis on plain radiography. A prospective, longitudinal study. J Rheumatol. 1999;26:1953–8. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

