Persistent Increase in Microglial RAGE Contributes to Chronic Stress-Induced Priming of Depressive-like Behavior
- PMID: 28882317
- PMCID: PMC6369917
- DOI: 10.1016/j.biopsych.2017.06.034
Persistent Increase in Microglial RAGE Contributes to Chronic Stress-Induced Priming of Depressive-like Behavior
Abstract
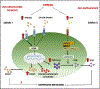
Background: Chronic stress-induced inflammatory responses occur in part via danger-associated molecular pattern (DAMP) molecules, such as high mobility group box 1 protein (HMGB1), but the receptor(s) underlying DAMP signaling have not been identified.
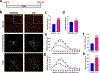
Methods: Microglia morphology and DAMP signaling in enriched rat hippocampal microglia were examined during the development and expression of chronic unpredictable stress (CUS)-induced behavioral deficits, including long-term, persistent changes after CUS.
Results: The results show that CUS promotes significant morphological changes and causes robust upregulation of HMGB1 messenger RNA in enriched hippocampal microglia, an effect that persists for up to 6 weeks after CUS exposure. This coincides with robust and persistent upregulation of receptor for advanced glycation end products (RAGE) messenger RNA, but not toll-like receptor 4 in hippocampal microglia. CUS also increased surface expression of RAGE protein on hippocampal microglia as determined by flow cytometry and returned to basal levels 5 weeks after CUS. Importantly, exposure to short-term stress was sufficient to increase RAGE surface expression as well as anhedonic behavior, reflecting a primed state that results from a persistent increase in RAGE messenger RNA expression. Further evidence for DAMP signaling in behavioral responses is provided by evidence that HMGB1 infusion into the hippocampus was sufficient to cause anhedonic behavior and by evidence that RAGE knockout mice were resilient to stress-induced anhedonia.
Conclusions: Together, the results provide evidence of persistent microglial HMGB1-RAGE expression that increases vulnerability to depressive-like behaviors long after chronic stress exposure.
Keywords: Depression; HMGB1; High mobility group box 1 protein; Microglia; RAGE; Receptor for advanced glycation end products; Stress; TLR4; toll-like receptor 4.
Copyright © 2017 Society of Biological Psychiatry. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
CONFLICT OF INTEREST
The authors declare no conflict of interest.
Figures

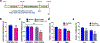



Comment in
-
Stress, Depression, and Inflammation: Molecular and Microglial Mechanisms.Biol Psychiatry. 2018 Jan 1;83(1):5-6. doi: 10.1016/j.biopsych.2017.10.025. Biol Psychiatry. 2018. PMID: 29173706 No abstract available.
References
-
- Moylan S, Maes M, Wray NR, Berk M (2013): The neuroprogressive nature of major depressive disorder: pathways to disease evolution and resistance, and therapeutic implications. Mol Psychiatry 18:595–606. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

