Polio immunity and the impact of mass immunization campaigns in the Democratic Republic of the Congo
- PMID: 28882442
- PMCID: PMC5628608
- DOI: 10.1016/j.vaccine.2017.08.063
Polio immunity and the impact of mass immunization campaigns in the Democratic Republic of the Congo
Abstract
Background: In order to prevent outbreaks from wild and vaccine-derived poliovirus, maintenance of population immunity in non-endemic countries is critical.
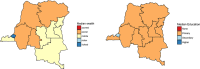
Methods: We estimated population seroprevalence using dried blood spots collected from 4893 children 6-59months olds in the 2013-2014 Demographic and Health Survey in the Democratic Republic of the Congo (DRC).
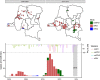
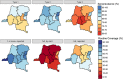
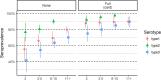
Results: Population immunity was 81%, 90%, and 70% for poliovirus types 1, 2, and 3, respectively. Among 6-59-month-old children, 78% reported at least one dose of polio in routine immunization, while only 15% had three doses documented on vaccination cards. All children in the study had been eligible for at least two trivalent oral polio vaccine campaigns at the time of enrollment; additional immunization campaigns seroconverted 5.0%, 14%, and 5.5% of non-immune children per-campaign for types 1, 2, and 3, respectively, averaged over relevant campaigns for each serotype.
Conclusions: Overall polio immunity was high at the time of the study, though pockets of low immunity cannot be ruled out. The DRC still relies on supplementary immunization campaigns, and this report stresses the importance of the quality and coverage of those campaigns over their quantity, as well as the importance of routine immunization.
Keywords: Democratic Republic of the Congo; Immunization; Mass vaccination; Poliomyelitis; Seroprevalence.
Copyright © 2017 The Authors. Published by Elsevier Ltd.. All rights reserved.
Figures



References
-
- Plotkin SA, Orenstein W, Offit PA. Poliovirus vaccine-live. Vaccines. 6th ed.; 2013, p. 598–645.
-
- World Health Organization. Polio this week 2017. <http://polioeradication.org/polio-today/polio-now/this-week/> [accessed May 26, 2017].
-
- Vidor E, Plotkin SA. Poliovirus vaccine - inactivated. Vaccines. 6th ed., Elsevier; n.d.
-
- Hampton L, Farrell M, Ramirez-Gonzalez A, Menning L, Shendale J, Lewis I, et al. Cessation of trivalent oral poliovirus vaccine and introduction of inactivated poliovirus vaccine — worldwide, 2016. Morb Mortal Wkly Rep 2016; 65: 934–8. doi:10.15585/mmwr.mm6535a3. - PubMed
-
- Burns C.C., Diop O.M., Sutter R.W., Kew O.M. Vaccine-derived polioviruses. J Infect Dis. 2014;210:S283–S293. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

