The impact of androgen actions in neurons on metabolic health and disease
- PMID: 28882554
- PMCID: PMC5835167
- DOI: 10.1016/j.mce.2017.09.001
The impact of androgen actions in neurons on metabolic health and disease
Abstract
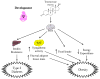
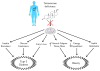
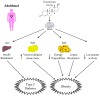
The male hormone testosterone exerts different effects on glucose and energy homeostasis in males and females. Testosterone deficiency predisposes males to visceral obesity, insulin resistance and type 2 diabetes. However, testosterone excess predisposes females to similar metabolic dysfunction. Here, we review the effects of testosterone actions in the central nervous system on metabolic function in males and females. In particular, we highlight changes within the hypothalamus that control glucose and energy homeostasis. We distinguish the organizational effects of testosterone in the programming of neural circuitry during development from the activational effects of testosterone during adulthood. Finally, we explore potential sites where androgen might be acting to impact metabolism within the central nervous system.
Keywords: Androgen receptor (AR); Arcuate nucleus (ARC); Central nervous system (CNS); Hypothalamus; Insulin resistance; Metabolism; Obesity; Polycystic ovarian syndrome (PCOS); Sex differences; Testosterone.
Copyright © 2017 Elsevier B.V. All rights reserved.
Figures
References
-
- Abbott D, Dumesic D, Franks S. Developmental origin of polycystic ovary syndrome-a hypothesis. J Endocrinol. 2002;174:1–5. JOE04772. - PubMed
-
- Alexanderson C, Eriksson E, Stener-Victorin E, Lystig T, Gabrielsson B, Lonn M, Holmang A. Postnatal testosterone exposure results in insulin resistance, enlarged mesenteric adipocytes, and an atherogenic lipid profile in adult female rats: Comparisons with estradiol and dihydrotestosterone. Endocrinology. 2007;148:5369–5376. doi: 10.1210/en.2007-0305. - DOI - PubMed
-
- Altman J, Bayer SA. The development of the rat hypothalamaus. Adv Anat Embryol Cell Biol. 1986;100:1–178. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

