Opsin 3 and 4 mediate light-induced pulmonary vasorelaxation that is potentiated by G protein-coupled receptor kinase 2 inhibition
- PMID: 28882814
- PMCID: PMC6335007
- DOI: 10.1152/ajplung.00091.2017
Opsin 3 and 4 mediate light-induced pulmonary vasorelaxation that is potentiated by G protein-coupled receptor kinase 2 inhibition
Abstract
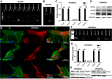
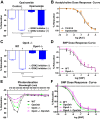
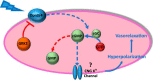
We recently demonstrated that blue light induces vasorelaxation in the systemic mouse circulation, a phenomenon mediated by the nonvisual G protein-coupled receptor melanopsin (Opsin 4; Opn4). Here we tested the hypothesis that nonvisual opsins mediate photorelaxation in the pulmonary circulation. We discovered Opsin 3 (Opn3), Opn4, and G protein-coupled receptor kinase 2 (GRK2) in rat pulmonary arteries (PAs) and in pulmonary arterial smooth muscle cells (PASMCs), where the opsins interact directly with GRK2, as demonstrated with a proximity ligation assay. Light elicited an intensity-dependent relaxation of PAs preconstricted with phenylephrine (PE), with a maximum response between 400 and 460 nm (blue light). Wavelength-specific photorelaxation was attenuated in PAs from Opn4-/- mice and further reduced following shRNA-mediated knockdown of Opn3. Inhibition of GRK2 amplified the response and prevented physiological desensitization to repeated light exposure. Blue light also prevented PE-induced constriction in isolated PAs, decreased basal tone, ablated PE-induced single-cell contraction of PASMCs, and reversed PE-induced depolarization in PASMCs when GRK2 was inhibited. The photorelaxation response was modulated by soluble guanylyl cyclase but not by protein kinase G or nitric oxide. Most importantly, blue light induced significant vasorelaxation of PAs from rats with chronic pulmonary hypertension and effectively lowered pulmonary arterial pressure in isolated intact perfused rat lungs subjected to acute hypoxia. These findings show that functional Opn3 and Opn4 in PAs represent an endogenous "optogenetic system" that mediates photorelaxation in the pulmonary vasculature. Phototherapy in conjunction with GRK2 inhibition could therefore provide an alternative treatment strategy for pulmonary vasoconstrictive disorders.
Keywords: GRK2; opsins; photorelaxation; pulmonary artery smooth muscle; pulmonary hypertension.
Figures







References
-
- An SS, Mitzner W, Tang W-Y, Ahn K, Yoon AR, Huang J, Kilic O, Yong HM, Fahey JW, Kumar S, Biswal S, Holgate ST, Panettieri RA Jr, Solway J, Liggett SB. An inflammation-independent contraction mechanophenotype of airway smooth muscle in asthma. J Allergy Clin Immunol 138: 294–297.e4, 2016. doi:10.1016/j.jaci.2015.12.1315. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

