Slow fusion pore expansion creates a unique reaction chamber for co-packaged cargo
- PMID: 28882880
- PMCID: PMC5694939
- DOI: 10.1085/jgp.201711842
Slow fusion pore expansion creates a unique reaction chamber for co-packaged cargo
Abstract
A lumenal secretory granule protein, tissue plasminogen activator (tPA), greatly slows fusion pore dilation and thereby slows its own discharge. We investigated another outcome of the long-lived narrow fusion pore: the creation of a nanoscale chemical reaction chamber for granule contents in which the pH is suddenly neutralized upon fusion. Bovine adrenal chromaffin cells endogenously express both tPA and its primary protein inhibitor, plasminogen activator inhibitor 1 (PAI). We found by immunocytochemistry that tPA and PAI are co-packaged in the same secretory granule. It is known that PAI irreversibly and covalently inactivates tPA at neutral pH. We demonstrate with zymography that the acidic granule lumen protects tPA from inactivation by PAI. Immunocytochemistry, total internal reflection fluorescence (TIRF) microscopy, and polarized TIRF microscopy demonstrated that co-packaged PAI and tPA remain together in granules for many seconds in the nanoscale reaction chamber, more than enough time to inhibit tPA and create a new secreted protein species.
© 2017 Bohannon et al.
Figures



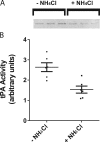
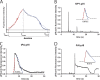

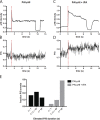
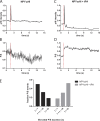
Comment in
-
Chemistry in a vesicle.J Gen Physiol. 2017 Oct 2;149(10):893-896. doi: 10.1085/jgp.201711894. Epub 2017 Sep 12. J Gen Physiol. 2017. PMID: 28899933 Free PMC article.
References
MeSH terms
Substances
Associated data
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

