The Strong Cell-based Hydrogen Peroxide Generation Triggered by Cold Atmospheric Plasma
- PMID: 28883477
- PMCID: PMC5589829
- DOI: 10.1038/s41598-017-11480-x
The Strong Cell-based Hydrogen Peroxide Generation Triggered by Cold Atmospheric Plasma
Abstract
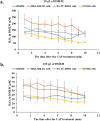
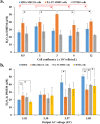
Hydrogen peroxide (H2O2) is an important signaling molecule in cancer cells. However, the significant secretion of H2O2 by cancer cells have been rarely observed. Cold atmospheric plasma (CAP) is a near room temperature ionized gas composed of neutral particles, charged particles, reactive species, and electrons. Here, we first demonstrated that breast cancer cells and pancreatic adenocarcinoma cells generated micromolar level H2O2 during just 1 min of direct CAP treatment on these cells. The cell-based H2O2 generation is affected by the medium volume, the cell confluence, as well as the discharge voltage. The application of cold atmospheric plasma (CAP) in cancer treatment has been intensively investigated over the past decade. Several cellular responses to CAP treatment have been observed including the consumption of the CAP-originated reactive species, the rise of intracellular reactive oxygen species, the damage on DNA and mitochondria, as well as the activation of apoptotic events. This is a new previously unknown cellular response to CAP, which provides a new prospective to understand the interaction between CAP and cells in vitro and in vivo. The short-lived reactive species in CAP may activate cells in vivo to generate long-lived reactive species such as H2O2, which may trigger immune attack on tumorous tissues via the H2O2-mediated lymphocyte activation.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures


References
-
- Szatrowski TP, Nathan CF. Production of large amounts of hydrogen peroxide by human tumor cells. Cancer. Res. 1991;51:794–799. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

