Global fibroblast activation throughout the left ventricle but localized fibrosis after myocardial infarction
- PMID: 28883544
- PMCID: PMC5589875
- DOI: 10.1038/s41598-017-09790-1
Global fibroblast activation throughout the left ventricle but localized fibrosis after myocardial infarction
Abstract
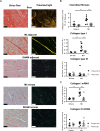
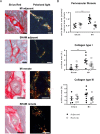
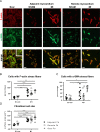
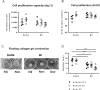
Fibroblast (Fb) differentiation and interstitial fibrosis contribute to cardiac remodeling and loss of function after myocardial infarction (MI). We investigated regional presence and regulation of fibrosis in a pig MI model. In vivo analysis of regional function and perfusion defined three regions: the scar, the myocardium adjacent to the scar (MIadjacent, reduced function, reduced perfusion reserve), and the remote myocardium (MIremote, minimal functional deficit, maintained perfusion). Interstitial and perivascular fibrosis, and increase of collagen type I, was only observed in the MIadjacent. Fb activated protein-alpha (FAP-α) was enriched in MIadjacent compared to MIremote. TGF-β1, which triggers Fb differentiation, was upregulated in both MIadjacent and MIremote, whereas lysyl oxidase, a regulator of collagen cross-linking, and the proteoglycans decorin and biglycan were only increased in the MIadjacent. Fb isolated and cultured for 4 days had myoFb characteristics with little difference between MIremote and MIadjacent, although RNA sequencing revealed differences in gene expression profiles. Fbs from all regions maintained proliferative capacity, and induced contraction of 3-D collagen matrices but scar myoFb was more effective. These data suggest that after MI, signaling through TGF-β1, possibly related to increased mechanical load, drives Fb activation throughout the left ventricle while regional signaling determines further maturation and extracellular matrix remodeling after MI.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

