Evidence that endogenous formaldehyde produces immunogenic and atherogenic adduct epitopes
- PMID: 28883613
- PMCID: PMC5589919
- DOI: 10.1038/s41598-017-11289-8
Evidence that endogenous formaldehyde produces immunogenic and atherogenic adduct epitopes
Abstract
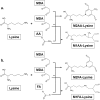
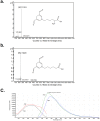
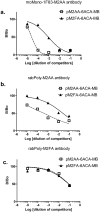
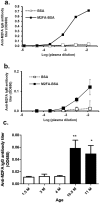
Endogenous formaldehyde is abundantly present in our bodies, at around 100 µM under normal conditions. While such high steady state levels of formaldehyde may be derived by enzymatic reactions including oxidative demethylation/deamination and myeloperoxidation, it is unclear whether endogenous formaldehyde can initiate and/or promote diseases in humans. Here, we show that fluorescent malondialdehyde-formaldehyde (M2FA)-lysine adducts are immunogenic without adjuvants in mice. Natural antibody titers against M2FA are elevated in atherosclerosis-prone mice. Staining with an antibody against M2FA demonstrated that M2FA is present in plaque found on the aortic valve of ApoE -/- mice. To mimic inflammation during atherogenesis, human myeloperoxidase was incubated with glycine, H2O2, malondialdehyde, and a lysine analog in PBS at a physiological temperature, which resulted in M2FA generation. These results strongly suggest that the 1,4-dihydropyridine-type of lysine adducts observed in atherosclerosis lesions are likely produced by endogenous formaldehyde and malondialdehyde with lysine. These highly fluorescent M2FA adducts may play important roles in human inflammatory and degenerative diseases.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures
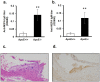
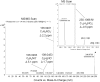
References
-
- Kikugawa K, Machida Y, Kida M, Kurechi T. Studies on Peroxidized Lipids. III. Fluorescent Pigments derived from the Reaction of Malonaldehyde and Amino AcidsNo Title. Chem. Pharm. Bull. (Tokyo). 1981;29:3003–3011. doi: 10.1248/cpb.29.3003. - DOI
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

