ACTIVATE: the effect of aclidinium/formoterol on hyperinflation, exercise capacity, and physical activity in patients with COPD
- PMID: 28883722
- PMCID: PMC5574699
- DOI: 10.2147/COPD.S143488
ACTIVATE: the effect of aclidinium/formoterol on hyperinflation, exercise capacity, and physical activity in patients with COPD
Abstract
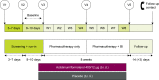
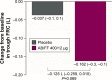
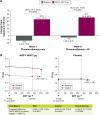
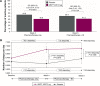
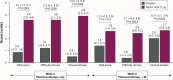
The Phase IV, 8-week, randomized, double-blind, placebo-controlled ACTIVATE study (NCT02424344) evaluated the effect of aclidinium/formoterol (AB/FF) 400/12 μg twice daily on lung hyperinflation, exercise capacity, and physical activity in patients with moderate-to-severe COPD. Patients received AB/FF (n=134) or placebo (n=133) (1:1) via the Genuair™/Pressair® dry powder inhaler for 8 weeks. From Weeks 5 to 8, all patients participated in behavioral intervention (BI; daily messages providing step goals). The primary end point was trough functional residual capacity (FRC) at Week 4. Exercise endurance time and physical activity were assessed at Week 4 (pharmacotherapy only) and at Week 8 (8 weeks of pharmacotherapy plus 4 weeks of BI). Other end points included post-dose FRC, residual volume, and inspiratory capacity (IC) at rest and during exercise. After 4 weeks, trough FRC improved with AB/FF versus placebo but did not reach significance (125 mL; P=0.0690). However, post-dose FRC, residual volume, and IC at rest improved significantly with AB/FF at Week 4 versus placebo (all P<0.0001). AB/FF significantly improved exercise endurance time and IC at isotime versus placebo at Week 4 (P<0.01 and P<0.0001, respectively) and Week 8 (P<0.05 and P<0.0001, respectively). AB/FF achieved higher step counts (P<0.01) with fewer inactive patients (P<0.0001) at Week 4 versus placebo. Following BI, AB/FF maintained improvements in physical activity at Week 8 and nonsignificant improvements were observed with placebo. AB/FF 400/12 μg demonstrated improvements in lung hyperinflation, exercise capacity, and physical activity versus placebo that were maintained following the addition of BI. A 4-week period of BI might be too short to augment the improvements of physical activity observed with AB/FF.
Keywords: COPD; aclidinium; exercise capacity; formoterol; hyperinflation; physical activity.
Conflict of interest statement
Disclosure Henrik Watz has received honoraria for consultancies, lectures, and travel support to attend scientific congresses from Almirall, AstraZeneca, Berlin-Chemie, Boehringer Ingelheim, Chiesi, GlaxoSmithKline, Novartis, and Takeda. His institution received investigator fees for participation in clinical trials from Almirall, AstraZeneca, Bayer Health Care, Berlin-Chemie, Boehringer Ingelheim, Chiesi, GlaxoSmithKline, Novartis, Sanofi Aventis, Roche, and Takeda. Thierry Troosters has received grants from the Innovative Medicines Initiative Joint Undertaking and speaker fees from Boehringer Ingelheim, AstraZeneca, and GlaxoSmithKline. Kai M Beeh’s institution has received compensation for organizing or participating in advisory boards (from Almirall Hermal, Cytos, Chiesi, Boehringer Ingelheim, AstraZeneca, Mundipharma, Novartis, and Teva), has participated in scientific meetings or courses (supported by Almirall Hermal, AstraZeneca, Boehringer Ingelheim, Mundipharma, Novartis, Pfizer, and Teva) in the past 3 years, has received consulting fees (from Ablynx, Apellis Pharmaceuticals, Sterna GmbH, Chiesi, and Cytos), and has received compensation for the design, performance, or participation in single or multicenter clinical trials in the past 3 years (from Almirall, AstraZeneca, Boehringer Ingelheim, GlaxoSmith-Kline, Infinity, Mundipharma, Novartis, Pfizer, Revotar Biopharmaceuticals, Sterna GmbH, Teva, and Zentiva). Judith Garcia-Aymerich’s institution has received consulting and lecture fees from AstraZeneca, and lecture fees from Esteve. Pierluigi Paggiaro is on the AstraZeneca, Boehringer Ingelheim, Chiesi, GlaxoSmithKline, and Novartis boards and has received lecture fees from AstraZeneca, Boehringer Ingelheim, Chiesi, GlaxoSmithKline, Laboratori Guidotti, Menarini, Mundipharma, Novartis, Sanofi, and Teva. Eduard Molins, Diana Jarreta, and Esther Garcia Gil are employees of AstraZeneca PLC, Barcelona, Spain. Massimo Notari is an employee of A. Menarini Farmaceutica Internazionale S.R.L., Firenze, Italy. Antonio Zapata is an employee of Laboratorios Menarini, S.A., Badalona, Spain. The authors report no other conflicts of interest in this work.
Figures
References
-
- Vogelmeier CF, Criner GJ, Martinez FJ, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive lung disease 2017 report: GOLD executive summary. Eur Respir J. 2017;49(3):1700214. - PubMed
-
- Watz H, Pitta F, Rochester CL, et al. An official European Respiratory Society statement on physical activity in COPD. Eur Respir J. 2014;44(6):1521–1537. - PubMed
-
- Shrikrishna D, Patel M, Tanner RJ, et al. Quadriceps wasting and physical inactivity in patients with COPD. Eur Respir J. 2012;40(5):1115–1122. - PubMed
-
- Watz H, Waschki B, Meyer T, Magnussen H. Physical activity in patients with COPD. Eur Respir J. 2009;33(2):262–272. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

