Functions of NQO1 in Cellular Protection and CoQ10 Metabolism and its Potential Role as a Redox Sensitive Molecular Switch
- PMID: 28883796
- PMCID: PMC5573868
- DOI: 10.3389/fphys.2017.00595
Functions of NQO1 in Cellular Protection and CoQ10 Metabolism and its Potential Role as a Redox Sensitive Molecular Switch
Abstract
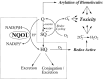
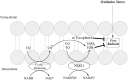
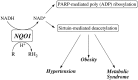
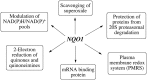
NQO1 is one of the two major quinone reductases in mammalian systems. It is highly inducible and plays multiple roles in cellular adaptation to stress. A prevalent polymorphic form of NQO1 results in an absence of NQO1 protein and activity so it is important to elucidate the specific cellular functions of NQO1. Established roles of NQO1 include its ability to prevent certain quinones from one electron redox cycling but its role in quinone detoxification is dependent on the redox stability of the hydroquinone generated by two-electron reduction. Other documented roles of NQO1 include its ability to function as a component of the plasma membrane redox system generating antioxidant forms of ubiquinone and vitamin E and at high levels, as a direct superoxide reductase. Emerging roles of NQO1 include its function as an efficient intracellular generator of NAD+ for enzymes including PARP and sirtuins which has gained particular attention with respect to metabolic syndrome. NQO1 interacts with a growing list of proteins, including intrinsically disordered proteins, protecting them from 20S proteasomal degradation. The interactions of NQO1 also extend to mRNA. Recent identification of NQO1 as a mRNA binding protein have been investigated in more detail using SERPIN1A1 (which encodes the serine protease inhibitor α-1-antitrypsin) as a target mRNA and indicate a role of NQO1 in control of translation of α-1-antitrypsin, an important modulator of COPD and obesity related metabolic syndrome. NQO1 undergoes structural changes and alterations in its ability to bind other proteins as a result of the cellular reduced/oxidized pyridine nucleotide ratio. This suggests NQO1 may act as a cellular redox switch potentially altering its interactions with other proteins and mRNA as a result of the prevailing redox environment.
Keywords: coenzyme Q; polymorphism; quinone reductases; quinones; single nucleotide; superoxide; vitamin E.
Figures

References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

