An Overview of Lipid Droplets in Cancer and Cancer Stem Cells
- PMID: 28883835
- PMCID: PMC5572636
- DOI: 10.1155/2017/1656053
An Overview of Lipid Droplets in Cancer and Cancer Stem Cells
Abstract
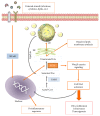
For decades, lipid droplets have been considered as the main cellular organelles involved in the fat storage, because of their lipid composition. However, in recent years, some new and totally unexpected roles have been discovered for them: (i) they are active sites for synthesis and storage of inflammatory mediators, and (ii) they are key players in cancer cells and tissues, especially in cancer stem cells. In this review, we summarize the main concepts related to the lipid droplet structure and function and their involvement in inflammatory and cancer processes.
Figures




References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

