Antibiotic resistance and biofilm formation among coagulase-negative staphylococci isolated from clinical samples at a tertiary care hospital of eastern Nepal
- PMID: 28883911
- PMCID: PMC5579930
- DOI: 10.1186/s13756-017-0251-7
Antibiotic resistance and biofilm formation among coagulase-negative staphylococci isolated from clinical samples at a tertiary care hospital of eastern Nepal
Abstract
Background: Coagulase negative staphylococci were long regarded non-pathogenic as they are the commensals of human skin and mucosa but the recent changes in the medical practice and changes in underlying host populations, they are being considered significant pathogens associated with number of nosocomial infections. The objective of the study was to determine the species, antimicrobial susceptibility pattern, biofilm forming ability of the clinically significant CoNS isolates and to compare the different methods for the detection of biofilm formation.
Methods: A total of 52 clinically significant CoNS isolates obtained from different units during a year period were studied. Characterization was done using standard microbiological guidelines and antimicrobial susceptibility was done following CLSI guidelines. Biofilm formation was detected by using three methods i.e. tissue culture plate method, congo red agar method and tube adherence method.
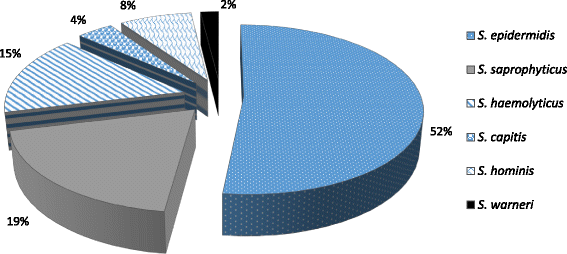
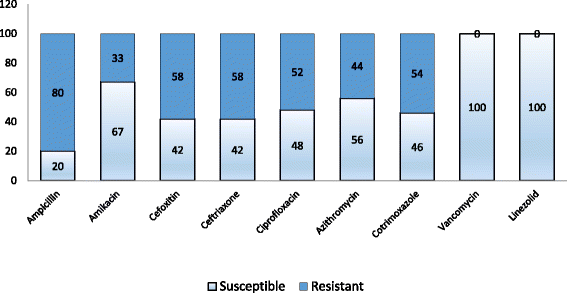
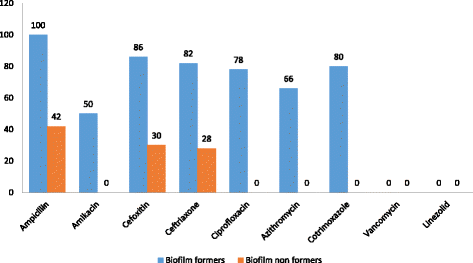
Results: Among 52 isolates, S. epidermidis (52%) was the most common species which was followed by S. saprophyticus (18%) and S. haemolyticus (14%). Antimicrobial susceptibility pattern of CoNS documented resistance of 80% to ampicillin. Resistance to cefoxitin and ceftriaxone was observed in 58% of the isolates. Biofilm formation was observed in 65.38% of the isolates. The accuracy of Congo red agar and tube adherence method for the detection of biofilm formation was 82% and 76% respectively.
Conclusion: CoNS isolates obtained from clinical samples should be processed routinely and antimicrobial susceptibility testing should be performed. Multidrug-resistant CoNS are prevalent. All the three methods i.e. tissue culture plate, Congo red agar and tube adherence method can be used in detecting biofilm formation.
Keywords: Biofilm; CoNS; Nosocomial infection.
Conflict of interest statement
Ethics approval and consent to participate
-was obtained from Institutional Ethical Review Board (IERB)
-code no IERB/196/014
-consent to participate: not applicable
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures
Similar articles
-
Phenotypic and genotypic characterization of biofilm producing clinical coagulase negative staphylococci from Nepal and their antibiotic susceptibility pattern.Ann Clin Microbiol Antimicrob. 2021 May 31;20(1):41. doi: 10.1186/s12941-021-00447-6. Ann Clin Microbiol Antimicrob. 2021. PMID: 34059077 Free PMC article.
-
Comparative evaluation of methods for the detection of biofilm formation in coagulase-negative staphylococci and correlation with antibiogram.Infect Drug Resist. 2018 Apr 24;11:607-613. doi: 10.2147/IDR.S159764. eCollection 2018. Infect Drug Resist. 2018. PMID: 29731649 Free PMC article.
-
[Investigation of Biofilm Formation Properties of Coagulase Negative Staphylococci Isolated from Catheter-Related Bloodstream Infections].Mikrobiyol Bul. 2022 Jul;56(3):506-524. doi: 10.5578/mb.20229710. Mikrobiyol Bul. 2022. PMID: 35960241 Turkish.
-
Phenotypic characteristics of coagulase-negative staphylococci: typing and antibiotic susceptibility.APMIS Suppl. 1999;91:1-42. APMIS Suppl. 1999. PMID: 10230367 Review.
-
Virulence Factors in Coagulase-Negative Staphylococci.Pathogens. 2021 Feb 4;10(2):170. doi: 10.3390/pathogens10020170. Pathogens. 2021. PMID: 33557202 Free PMC article. Review.
Cited by
-
Antimicrobial resistance patterns and biofilm formation of coagulase-negative Staphylococcus species isolated from subclinical mastitis cow milk samples submitted to the Onderstepoort Milk Laboratory.BMC Vet Res. 2019 Nov 26;15(1):420. doi: 10.1186/s12917-019-2175-3. BMC Vet Res. 2019. PMID: 31771575 Free PMC article.
-
Comparative study of antimicrobial resistance and biofilm formation among Gram-positive uropathogens isolated from community-acquired urinary tract infections and catheter-associated urinary tract infections.Infect Drug Resist. 2019 Apr 23;12:957-963. doi: 10.2147/IDR.S200988. eCollection 2019. Infect Drug Resist. 2019. PMID: 31118702 Free PMC article.
-
Coagulase-negative staphylococci from bovine milk: Antibiogram profiles and virulent gene detection.BMC Microbiol. 2024 Jul 18;24(1):263. doi: 10.1186/s12866-024-03415-0. BMC Microbiol. 2024. PMID: 39026151 Free PMC article.
-
Evaluation of methods to detect in vitro biofilm formation by staphylococcal clinical isolates.BMC Res Notes. 2018 Oct 10;11(1):714. doi: 10.1186/s13104-018-3820-9. BMC Res Notes. 2018. PMID: 30305150 Free PMC article.
-
The species distribution, antimicrobial resistance and risk factors for poor outcome of coagulase-negative staphylococci bacteraemia in China.Antimicrob Resist Infect Control. 2019 Apr 24;8:65. doi: 10.1186/s13756-019-0523-5. eCollection 2019. Antimicrob Resist Infect Control. 2019. PMID: 31044070 Free PMC article.
References
-
- Bannerman TL. Staphylococcus, micrococcus, and other catalasepositive cocci that grow aerobically. In: Murray PR, Baron EJ, Jorgensen JH, editors. Manual of medical microbiology. Washington, DC: American Society For Microbiology; 2003. pp. 384–404.
-
- Rupp ME, Fey PD. Staphylococcus Epidermidis and other Coagulase negative staphylococci. In: Mandell GL, Benett JE, Dolin R, editors. Mandell, Douglas and Bennett’s principles and practice of infectious disease. 7th. Philadelphia: Churchill Livingstone Elsevier; 2010. p. 186.
LinkOut - more resources
Full Text Sources
Other Literature Sources