Effect of recombinant and native buffalo OVGP1 on sperm functions and in vitro embryo development: a comparative study
- PMID: 28883914
- PMCID: PMC5580196
- DOI: 10.1186/s40104-017-0201-5
Effect of recombinant and native buffalo OVGP1 on sperm functions and in vitro embryo development: a comparative study
Abstract
Background: An oviduct- specific glycoprotein, OVGP1, is synthesized and secreted by non-ciliated epithelial cells of the mammalian oviduct which provides an essential milieu for reproductive functions. The present study reports the effects of recombinant buffalo OVGP1 that lacks post-translational modifications, and native Buffalo OVGP1 isolated from oviductal tissue, on frozen- thawed sperm functions and in vitro embryo development.
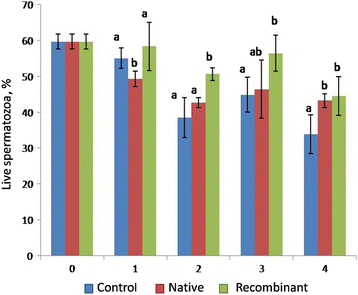
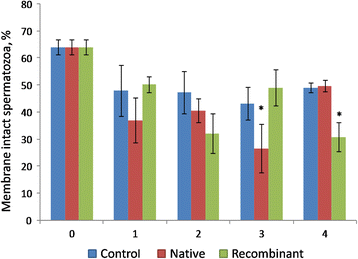
Results: The proportion of viable sperms was greater (P < 0.05) in the recombinant OVGP1-treated group compared to the native OVGP1-treated group at 2 h, 3 h, and 4 h of incubation. The proportion of motile sperms at 3 h and 4 h of incubation; and membrane- intact sperms at 4 h was greater (P < 0.05) in the native OVGP1-treated group compared to the control and recombinant OVGP1-treated groups. The proportion of capacitated and acrosome- reacted sperms was greater (P < 0.05) in the native OVGP1-treated group compared to the recombinant OVGP1 group at 4 h. The rates of cleavage of embryos and their development to the blastocyst stage were greater (P < 0.05) in the presence of either native or recombinant OVGP1 in comparison to control at 10 μg/mL concentration as compared to 5 or 20 μg/mL.
Conclusions: The study suggests that both native and recombinant OVGP1 impart a positive effect on various sperm features and in vitro embryo development. However, native OVGP1 was found to have a more pronounced effect in comparison to recombinant non-glycosylated OVGP1 on various sperm functions except viability. Hence, our current findings infer that glycosylation of OVGP1 might be essential in sustaining the sperm functions but not the in vitro embryo development.
Keywords: Blastocyst; Capacitation; Fertilization; Glycoprotein; In vitro fertilization; Oestrus; Semen; Sperm.
Conflict of interest statement
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Figures
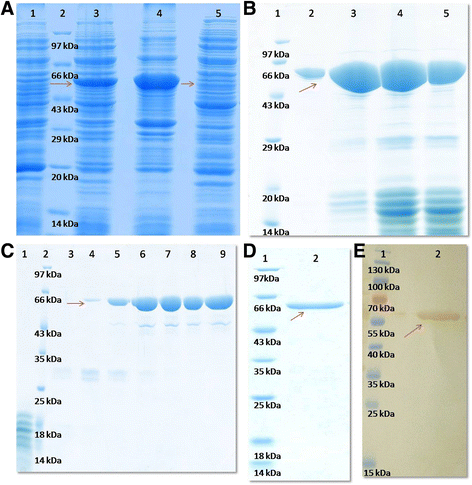
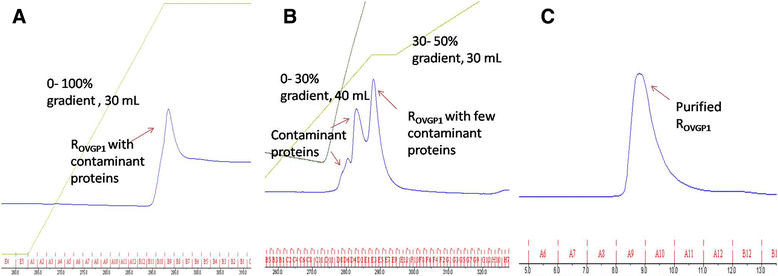
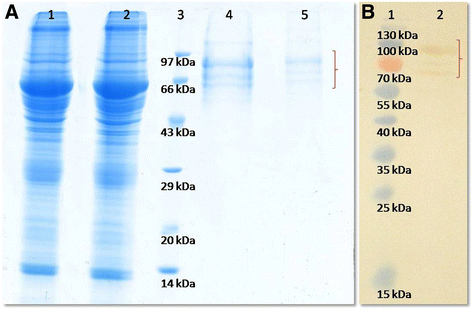
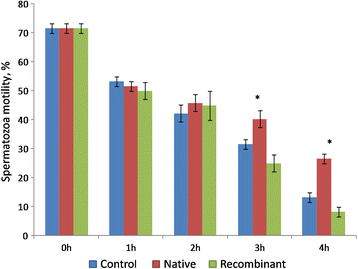


References
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

