The Pertussis resurgence: putting together the pieces of the puzzle
- PMID: 28883970
- PMCID: PMC5530967
- DOI: 10.1186/s40794-016-0043-8
The Pertussis resurgence: putting together the pieces of the puzzle
Abstract
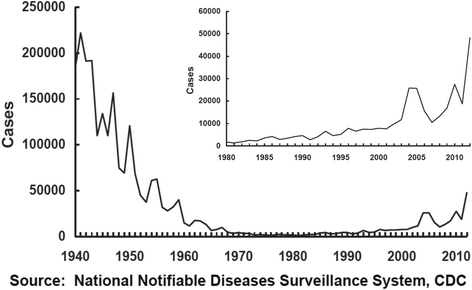
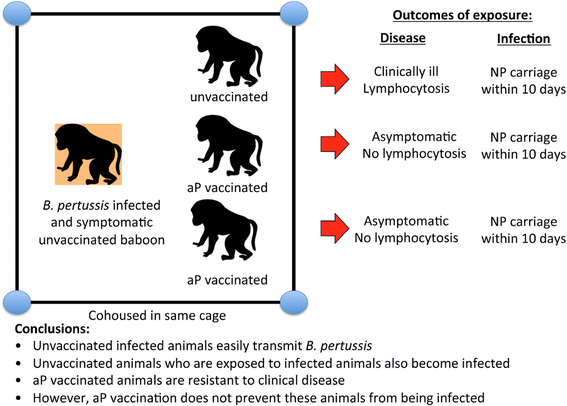
Pertussis incidence is rising in almost every country where acellular pertussis (aP) vaccines have been introduced, and is occurring across all age groups from infancy to adulthood. The key question is why? While several known factors such as waning of immunity, detection bias due to more sensitive tests and higher awareness of the disease among practitioners, and evolutionary shifts among B. pertussis all likely contribute, collectively, these do not adequately explain the existing epidemiologic data, suggesting that additional factors also contribute. Key amongst these is recent data indicating that the immune responses induced by aP vaccines differ fundamentally from those induced by the whole cell pertussis (wP) vaccines, and do not lead to mucosal immunity. If so, it appears likely that differences in how the two categories of vaccines work, may be pivotal to our overall understanding of the pertussis resurgence.
Keywords: Acellular pertussis vaccine; Asymptomatic transmission; Epidemiologic modeling; Pertussis; Pertussis vaccines; Resurgence; Review.
Figures
References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

