Vaccination of HIV-infected pregnant women: implications for protection of their young infants
- PMID: 28883971
- PMCID: PMC5530931
- DOI: 10.1186/s40794-016-0044-7
Vaccination of HIV-infected pregnant women: implications for protection of their young infants
Abstract
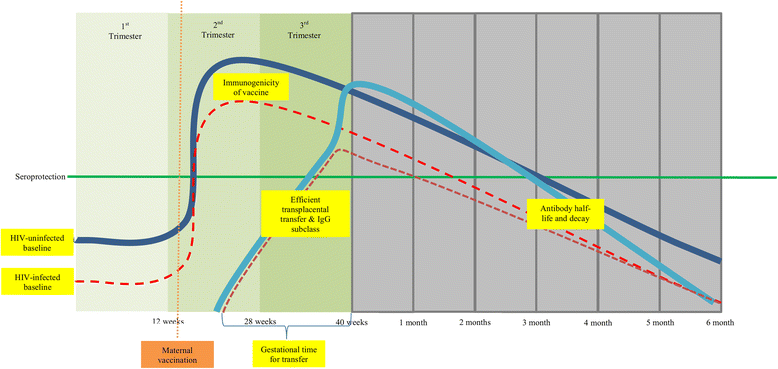
Background: The prevention of mother to child transmission of HIV has resulted in reduced burden of pediatric HIV-infection, but the prevalence of maternal HIV infection remains high in sub-Saharan African countries. HIV-exposed-uninfected infants have an increased risk of morbidity and mortality due to infectious diseases than HIV-unexposed infants, particularly during the first six months of life, which in part might be due to lower levels of pathogen-specific protective antibodies acquired transplacentally from their mothers. This could be mitigated by vaccinating pregnant women to boost antibody levels; although vaccine responses among HIV-infected pregnant women might differ compared to HIV-uninfected women. We reviewed studies that compared natural and vaccine-induced antibody levels to different epitopes between HIV-infected and HIV-uninfected pregnant women.
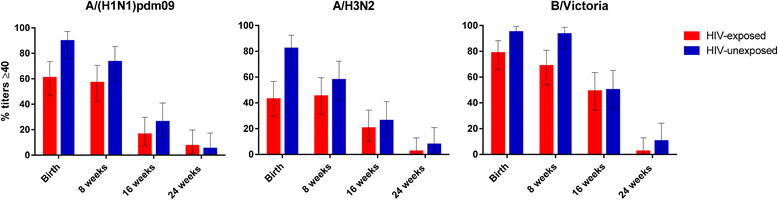
Findings: Most studies reported lower baseline/pre-vaccination antibody levels in HIV-infected pregnant women, which may not be reversed by antiretroviral therapy during pregnancy. There were only few studies on vaccination of HIV-infected pregnant women, mainly on influenza virus and group B Streptococcus (GBS) vaccines. Immunogenicity studies on influenza vaccines indicated that HIV-infected pregnant women had lower vaccine induced hemagglutination inhibition antibody titers and a decreased likelihood of seroconversion compared to HIV-uninfected women; and while higher CD4+ T-lymphocyte levels were associated with better immune responses to vaccination, HIV viral load was not associated with responses. Furthermore, infants born to influenza vaccinated HIV-infected pregnant women also had lower antibody levels and a lower proportion of HIV-exposed infants had titers above the putative correlate of protection compared to HIV-unexposed infants. The immunogenicity of a CRM197-conjugated trivalent GBS vaccine was also lower in HIV-infected pregnant women compared to HIV-uninfected women, irrespective of CD4+ T-lymphocyte counts.
Conclusions: Poorer immunogenicity of vaccines reported in HIV-infected compared to HIV-uninfected pregnant women might compromise the potential benefits to their young infants. Alternate vaccination strategies, including vaccines with higher antigen concentration, adjuvanted vaccines or multiple doses schedules might be required in HIV-infected pregnant women to optimize antibody transferred to their fetuses.
Keywords: Antibody; HIV; Immunity; Pregnant women; Vaccine.
Figures
References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

