Redox-active conducting polymers modulate Salmonella biofilm formation by controlling availability of electron acceptors
- PMID: 28883986
- PMCID: PMC5583241
- DOI: 10.1038/s41522-017-0027-0
Redox-active conducting polymers modulate Salmonella biofilm formation by controlling availability of electron acceptors
Erratum in
-
Erratum: Author Correction: Redox-active conducting polymers modulate Salmonella biofilm formation by controlling availability of electron acceptors.NPJ Biofilms Microbiomes. 2018 Aug 7;4:19. doi: 10.1038/s41522-018-0061-6. eCollection 2018. NPJ Biofilms Microbiomes. 2018. PMID: 30109118 Free PMC article.
Abstract
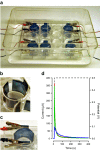
Biofouling is a major problem caused by bacteria colonizing abiotic surfaces, such as medical devices. Biofilms are formed as the bacterial metabolism adapts to an attached growth state. We studied whether bacterial metabolism, hence biofilm formation, can be modulated in electrochemically active surfaces using the conducting conjugated polymer poly(3,4-ethylenedioxythiophene) (PEDOT). We fabricated composites of PEDOT doped with either heparin, dodecyl benzene sulfonate or chloride, and identified the fabrication parameters so that the electrochemical redox state is the main distinct factor influencing biofilm growth. PEDOT surfaces fitted into a custom-designed culturing device allowed for redox switching in Salmonella cultures, leading to oxidized or reduced electrodes. Similarly large biofilm growth was found on the oxidized anodes and on conventional polyester. In contrast, biofilm was significantly decreased (52-58%) on the reduced cathodes. Quantification of electrochromism in unswitched conducting polymer surfaces revealed a bacteria-driven electrochemical reduction of PEDOT. As a result, unswitched PEDOT acquired an analogous electrochemical state to the externally reduced cathode, explaining the similarly decreased biofilm growth on reduced cathodes and unswitched surfaces. Collectively, our findings reveal two opposing effects affecting biofilm formation. While the oxidized PEDOT anode constitutes a renewable electron sink that promotes biofilm growth, reduction of PEDOT by a power source or by bacteria largely suppresses biofilm formation. Modulating bacterial metabolism using the redox state of electroactive surfaces constitutes an unexplored method with applications spanning from antifouling coatings and microbial fuel cells to the study of the role of bacterial respiration during infection.
Conflict of interest statement
The authors declare that they have no competing financial interests.
Figures



Similar articles
-
High-Resolution Large-Area Image Analysis Deciphers the Distribution of Salmonella Cells and ECM Components in Biofilms Formed on Charged PEDOT:PSS Surfaces.Adv Sci (Weinh). 2024 Jul;11(27):e2307322. doi: 10.1002/advs.202307322. Epub 2024 Jan 15. Adv Sci (Weinh). 2024. PMID: 38225703 Free PMC article.
-
A Graphene/Poly(3,4-ethylenedioxythiophene) Hybrid as an Anode for High-Performance Microbial Fuel Cells.Chempluschem. 2013 Aug;78(8):823-829. doi: 10.1002/cplu.201300102. Epub 2013 Jun 21. Chempluschem. 2013. PMID: 31986676
-
Electroenhanced Antimicrobial Coating Based on Conjugated Polymers with Covalently Coupled Silver Nanoparticles Prevents Staphylococcus aureus Biofilm Formation.Adv Healthc Mater. 2017 Oct;6(20). doi: 10.1002/adhm.201700435. Epub 2017 Aug 14. Adv Healthc Mater. 2017. PMID: 28805046
-
Synthesis and electrochemical sensing application of poly(3,4-ethylenedioxythiophene)-based materials: A review.Anal Chim Acta. 2018 Aug 31;1022:1-19. doi: 10.1016/j.aca.2018.02.080. Epub 2018 Mar 14. Anal Chim Acta. 2018. PMID: 29729729 Review.
-
Poly(3,4-ethylenedioxythiophene) (PEDOT) Derivatives: Innovative Conductive Polymers for Bioelectronics.Polymers (Basel). 2017 Aug 11;9(8):354. doi: 10.3390/polym9080354. Polymers (Basel). 2017. PMID: 30971030 Free PMC article. Review.
Cited by
-
Multi-omics analysis reveals genes and metabolites involved in Streptococcus suis biofilm formation.BMC Microbiol. 2024 Aug 10;24(1):297. doi: 10.1186/s12866-024-03448-5. BMC Microbiol. 2024. PMID: 39127666 Free PMC article.
-
Salmonella and Reactive Oxygen Species: A Love-Hate Relationship.J Innate Immun. 2019;11(3):216-226. doi: 10.1159/000496370. Epub 2019 Apr 3. J Innate Immun. 2019. PMID: 30943492 Free PMC article. Review.
-
Colonization of nasal cavities by Staphylococcus epidermidis mitigates SARS-CoV-2 nucleocapsid phosphoprotein-induced interleukin (IL)-6 in the lung.Microb Biotechnol. 2022 Jul;15(7):1984-1994. doi: 10.1111/1751-7915.13994. Epub 2022 Apr 14. Microb Biotechnol. 2022. PMID: 35426250 Free PMC article.
-
Recent Advances in the Control of Clinically Important Biofilms.Int J Mol Sci. 2022 Aug 23;23(17):9526. doi: 10.3390/ijms23179526. Int J Mol Sci. 2022. PMID: 36076921 Free PMC article. Review.
-
Research progress of stimulus-responsive antibacterial materials for bone infection.Front Bioeng Biotechnol. 2022 Dec 23;10:1069932. doi: 10.3389/fbioe.2022.1069932. eCollection 2022. Front Bioeng Biotechnol. 2022. PMID: 36636700 Free PMC article. Review.
References
-
- Wang H, Zhang X, Dong Y, Xu X, Zhou G. Insights into the transcriptome profile of mature biofilm of SalmonellaTyphimurium on stainless steels surface. Food Res. Int. 2015;77:378–384. doi: 10.1016/j.foodres.2015.08.034. - DOI
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

