Effects of hypergravity on gene levels in anti-gravity muscle and bone through the vestibular system in mice
- PMID: 28884429
- PMCID: PMC10717783
- DOI: 10.1007/s12576-017-0566-4
Effects of hypergravity on gene levels in anti-gravity muscle and bone through the vestibular system in mice
Abstract
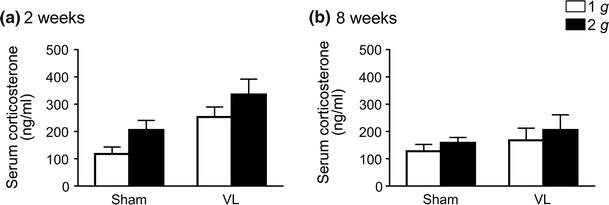
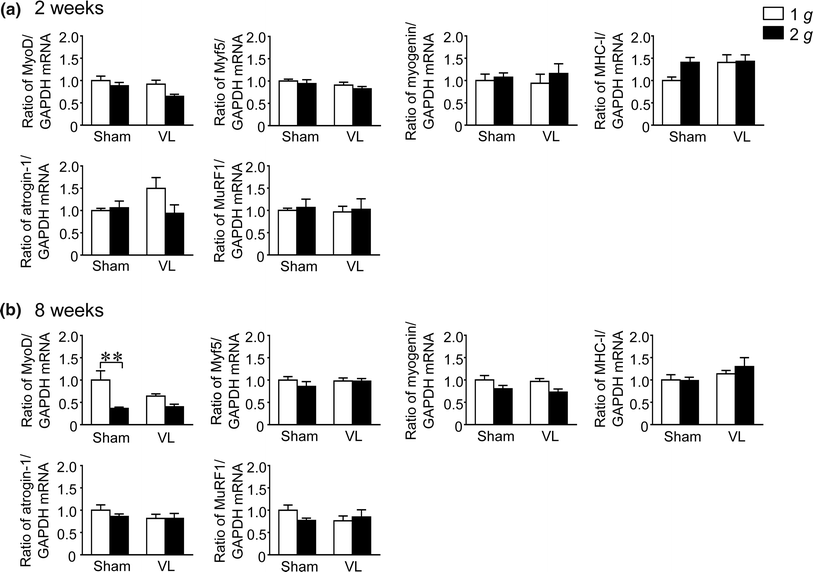
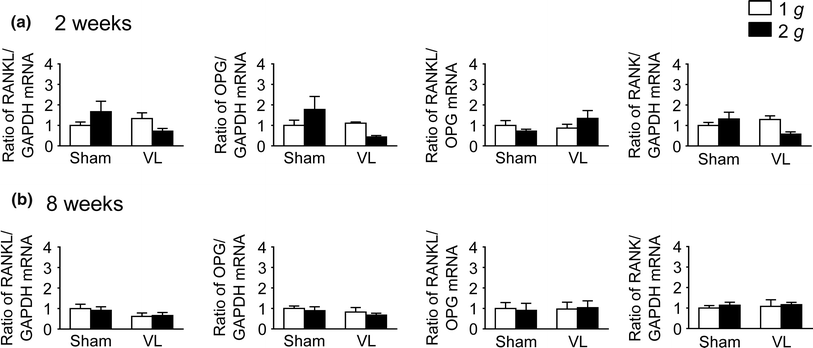
We recently reported that hypergravity with 3 g for 4 weeks affects muscle and bone through the vestibular system in mice. The purpose of this study was to investigate the effects of hypergravity with 2 g, which had no influence on circulating glucocorticoid level, on the gene levels in muscle and bone, as well as the roles of the vestibular system in those changes using vestibular lesioned (VL) mice. Hypergravity for 2 and 8 weeks or VL exerted little effects on the mRNA levels of muscle differentiation factors and myokines in the soleus muscle. Although hypergravity for 2 weeks significantly elevated alkaline phosphatase (ALP) and type I collagen mRNA levels in the tibia, VL significantly attenuated the levels of ALP mRNA enhanced by hypergravity. In conclusion, the present study suggests that a 2-g load for 2 weeks enhances osteoblast differentiation partly through the vestibular system in mice.
Keywords: Bone; Gravity change; Muscle; Vestibular system.
Conflict of interest statement
The authors declare that they have no conflicts of interest.
Figures

Similar articles
-
Understanding vestibular-related physiological functions could provide clues on adapting to a new gravitational environment.J Physiol Sci. 2020 Mar 14;70(1):17. doi: 10.1186/s12576-020-00744-3. J Physiol Sci. 2020. PMID: 32169037 Free PMC article. Review.
-
The vestibular system is critical for the changes in muscle and bone induced by hypergravity in mice.Physiol Rep. 2016 Oct;4(19):e12979. doi: 10.14814/phy2.12979. Physiol Rep. 2016. PMID: 27697847 Free PMC article.
-
Role of follistatin in muscle and bone alterations induced by gravity change in mice.J Cell Physiol. 2018 Feb;233(2):1191-1201. doi: 10.1002/jcp.25986. Epub 2017 Jun 22. J Cell Physiol. 2018. PMID: 28471505
-
Roles of Olfactomedin 1 in Muscle and Bone Alterations Induced by Gravity Change in Mice.Calcif Tissue Int. 2020 Aug;107(2):180-190. doi: 10.1007/s00223-020-00710-6. Epub 2020 May 27. Calcif Tissue Int. 2020. PMID: 32462291
-
Responses across the gravity continuum: hypergravity to microgravity.Adv Space Biol Med. 2005;10:225-45. doi: 10.1016/s1569-2574(05)10009-4. Adv Space Biol Med. 2005. PMID: 16101110 Review.
Cited by
-
Hypergravity load-induced hyperglycemia occurs due to hypothermia and increased plasma corticosterone level in mice.J Physiol Sci. 2022 Aug 1;72(1):18. doi: 10.1186/s12576-022-00844-2. J Physiol Sci. 2022. PMID: 35915429 Free PMC article.
-
Understanding vestibular-related physiological functions could provide clues on adapting to a new gravitational environment.J Physiol Sci. 2020 Mar 14;70(1):17. doi: 10.1186/s12576-020-00744-3. J Physiol Sci. 2020. PMID: 32169037 Free PMC article. Review.
-
Gravitational Experimental Platform for Animal Models, a New Platform at ESA's Terrestrial Facilities to Study the Effects of Micro- and Hypergravity on Aquatic and Rodent Animal Models.Int J Mol Sci. 2021 Mar 15;22(6):2961. doi: 10.3390/ijms22062961. Int J Mol Sci. 2021. PMID: 33803957 Free PMC article. Review.
-
Hypergravity and microgravity exhibited reversal effects on the bone and muscle mass in mice.Sci Rep. 2019 Apr 29;9(1):6614. doi: 10.1038/s41598-019-42829-z. Sci Rep. 2019. PMID: 31036903 Free PMC article.
-
Study of mouse behavior in different gravity environments.Sci Rep. 2021 Jan 29;11(1):2665. doi: 10.1038/s41598-021-82013-w. Sci Rep. 2021. PMID: 33514775 Free PMC article.
References
-
- Fitts RH, Trappe SW, Costill DL, Gallagher PM, Creer AC, Colloton PA, Peters JR, Romatowski JG, Bain JL, Riley DA. Prolonged space flight-induced alterations in the structure and function of human skeletal muscle fibres. J Physiol. 2010;588:3567–3592. doi: 10.1113/jphysiol.2010.188508. - DOI - PMC - PubMed
MeSH terms
Grants and funding
- 15K08220/Ministry of Education, Culture, Sports, Science and Technology
- 16K08534/Ministry of Education, Culture, Sports, Science and Technology
- 15K11916/Ministry of Education, Culture, Sports, Science and Technology
- 15YPTK-002009/Ministry of Education, Culture, Sports, Science and Technology
- 15H05935/Ministry of Education, Culture, Sports, Science and Technology
LinkOut - more resources
Full Text Sources
Other Literature Sources

