Ex vivo model unravelling cell distribution effect in hydrogels for cartilage repair
- PMID: 28884783
- PMCID: PMC7116182
- DOI: 10.14573/altex.1704171
Ex vivo model unravelling cell distribution effect in hydrogels for cartilage repair
Abstract
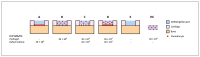
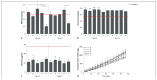
The implantation of chondrocyte-laden hydrogels is a promising cartilage repair strategy. Chondrocytes can be spatially positioned in hydrogels and thus in defects, while current clinical cell therapies introduce chondrocytes in the defect depth. The main aim of this study was to evaluate the effect of spatial chondrocyte distribution on the reparative process. To reduce animal experiments, an ex vivo osteochondral plug model was used and evaluated. The role of the delivered and endogenous cells in the repair process was investigated. Full thickness cartilage defects were created in equine osteochondral plugs. Defects were filled with (A) chondrocytes at the bottom of the defect, covered with a cell-free hydrogel, (B) chondrocytes homogeneously encapsulated in a hydrogel, and (C, D) combinations of A and B with different cell densities. Plugs were cultured for up to 57 days, after which the cartilage and repair tissues were characterized and compared to baseline samples. Additionally, at day 21, the origin of cells in the repair tissue was evaluated. Best outcomes were obtained with conditions C and D, which resulted in well-integrated cartilage-like tissue that completely filled the defect, regardless of the initial cell density. A critical role of the spatial chondrocyte distribution in the repair process was observed. Moreover, the osteochondral plugs stimulated cartilage formation in the hydrogels when cultured in the defects. The resulting repair tissue originated from the delivered cells. These findings confirm the potential of the osteochondral plug model for the optimization of the composition of cartilage implants and for studying repair mechanisms.
Keywords: GelMA/gellan gum; cartilage repair; osteochondral plug; regeneration.
Conflict of interest statement
The authors have no conflicts of interest to declare.
Figures





References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

