Certolizumab pegol (CDP870) for rheumatoid arthritis in adults
- PMID: 28884785
- PMCID: PMC6483724
- DOI: 10.1002/14651858.CD007649.pub4
Certolizumab pegol (CDP870) for rheumatoid arthritis in adults
Abstract
Background: Tumour necrosis factor (TNF)-alpha inhibitors are beneficial for the treatment of rheumatoid arthritis (RA) for reducing the risk of joint damage, improving physical function and improving the quality of life. This review is an update of the 2014 Cochrane Review of the treatment of RA with certolizumab pegol.
Objectives: To assess the clinical benefits and harms of certolizumab pegol (CZP) in people with RA who have not responded well to conventional disease-modifying anti-rheumatic drugs (DMARDs).
Search methods: We searched the Cochrane Central Register of Controlled Trials (CENTRAL: Cochrane Library 2016, Issue 9), MEDLINE, Embase, Web of Knowledge, reference lists of articles, clinicaltrials.gov and ICTRP of WHO. The searches were updated from 2014 (date of the last search for the previous version) to 26 September 2016.
Selection criteria: Randomised controlled trials that compared certolizumab pegol with any other agent, including placebo or methotrexate (MTX), in adults with active RA, regardless of current or prior treatment with conventional disease-modifying anti-rheumatic drugs (DMARDs), such as MTX.
Data collection and analysis: Two review authors independently checked search results, extracted data and assessed trial quality. We resolved disagreements by discussion or referral to a third review author.
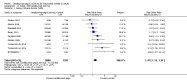
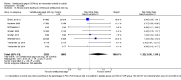
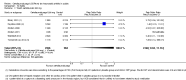
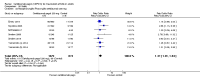
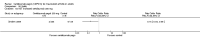
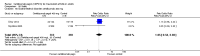
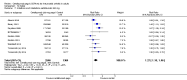
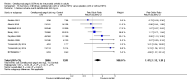
Main results: We included 14 trials in this update, three more than previously. Twelve trials (5422 participants) included measures of benefit. We pooled 11 of them, two more than previously. Thirteen trials included information on harms, (5273 participants). The duration of follow-up varied from 12 to 52 weeks and the range of doses of certolizumab pegol varied from 50 to 400 mg given subcutaneously. In Phase III trials, the comparator was placebo plus MTX in seven trials and placebo in five. In the two Phase II trials the comparator was only placebo.The approved dose of certolizumab pegol, 200 mg every other week, produced clinically important improvements at 24 weeks for the following outcomes:- American College of Rheumatology (ACR) 50% improvement (pain, function and other symptoms of RA): 25% absolute improvement (95% confidence interval (CI) 20% to 33%); number need to treat for an additional beneficial outcome (NNTB) of 4 (95% CI 3 to 5); risk ratio (RR) 3.80 (95% CI 2.42 to 5.95), 1445 participants, 5 studies.- The Health Assessment Questionnaire (HAQ): -12% absolute improvement (95% CI -9% to -14%); NNTB of 8 (95% CI 7 to 11); mean difference (MD) - 0.35 (95% CI -0.43 to -0.26; 1268 participants, 4 studies) (scale 0 to 3; lower scores mean better function).- Proportion of participants achieving remission (Disease Activity Score (DAS) < 2.6) absolute improvement 10% (95% CI 8% to 16%); NNTB of 8 (95% CI 6 to 12); risk ratio (RR) 2.94 (95% CI 1.64 to 5.28), 2420 participants, six studies.- Radiological changes: erosion score (ES) absolute improvement -0.29% (95% CI -0.42% to -0.17%); NNTB of 6 (95% CI 4 to 10); MD -0.67 (95% CI -0.96 to -0.38); 714 participants, two studies (scale 0 to 230), but not a clinically important difference.-Serious adverse events (SAEs) were statistically but not clinically significantly more frequent for certolizumab pegol (200 mg every other week) with an absolute rate difference of 3% (95% CI 1% to 4%); number needed to treat for an additional harmful outcome (NNTH) of 33 (95% CI 25 to 100); Peto odds ratio (OR) 1.47 (95% CI 1.13 to 1.91); 3927 participants, nine studies.There was a clinically significant increase in all withdrawals in the placebo groups (for all doses and at all follow-ups) with an absolute rate difference of -29% (95% CI -16% to -42%), NNTH of 3 (95% CI 2 to 6), RR 0.47 (95% CI 0.39 to 0.56); and there was a clinically significant increase in withdrawals due to adverse events in the certolizumab groups (for all doses and at all follow-ups) with an absolute rate difference of 2% (95% CI 0% to 3%); NNTH of 58 (95% CI 28 to 329); Peto OR 1.45 (95% CI 1.09 to 1.94) 5236 participants Twelve studies.We judged the quality of evidence to be high for ACR50, DAS remission, SAEs and withdrawals due to adverse events, and moderate for HAQ and radiological changes, due to concerns about attrition bias. For all withdrawals we judged the quality of evidence to be moderate, due to inconsistency.
Authors' conclusions: The results and conclusions did not change from the previous review. There is a moderate to high certainty of evidence from randomised controlled trials that certolizumab pegol, alone or combined with methotrexate, is beneficial in the treatment of RA for improved ACR50 and health-related quality of life, an increased chance of remission of RA, and reduced joint damage as seen on x-ray. Fewer people stopped taking their treatment, but most of these who did stopped due to serious adverse events. Adverse events were more frequent with active treatment. We found a clinically but not statistically significant risk of serious adverse events.
Conflict of interest statement
UCB paid Dr Vicente Ruiz's registration for the Cochrane meeting in Madrid 2011. In 2011 and 2012 he attended the UCB Advisory Board meetings in Madrid when the sponsor explained details and preliminary results for the new trials of certolizumab pegol. He did not receive any economic or other kind of compensation for these meetings.
Burls A: none known.
Cabello JB: none known.
Vela Casasempere P: "I have participated as a member of advisory boards for Roche and Pfizer. I have also received fees for development of educational presentations for Roche, Abbvie, UCB, BMS and MSD, and travel and accommodations expenses to attend scientific meetings from Pfizer, Abbvie and Roche".
Bort‐Marti S: none known.
Bernal JA: "I have received travel and accommodations expenses to attend scientific meetings from Pfizer and MSD".
Figures














































































































































































































Update of
-
Certolizumab pegol (CDP870) for rheumatoid arthritis in adults.Cochrane Database Syst Rev. 2014 Sep 18;(9):CD007649. doi: 10.1002/14651858.CD007649.pub3. Cochrane Database Syst Rev. 2014. Update in: Cochrane Database Syst Rev. 2017 Sep 08;9:CD007649. doi: 10.1002/14651858.CD007649.pub4. PMID: 25231904 Updated.
References
References to studies included in this review
Atsumi 2016 {published data only}
-
- Atsumi T, Yamamoto K, Takeuchi T, Yamanaka H, Ishiguro N, Tanaka Y, et al. Baseline parameters identified in early, methotrexate‐naive rheumatoid arthritis patients with better outcomes with certolizumab pegol+methotrexate compared to placebo+methotrexate: Post‐hoc analyses of c‐opera, a randomized, controlled, phase 3 study. Annals of the Rheumatic Diseases.Conference: Annual European Congress of Rheumatology of the European League Against Rheumatism, EULAR 2015 Rome Italy. 10 ‐ 13 June 2015. 2015; Vol. 74:716‐7.
-
- Atsumi T, Yamamoto K, Takeuchi T, Yamanaka H, Ishiguro N, Tanaka Y, et al. Clinical benefit of 1‐year certolizumab pegol treatment in MTX‐naïve, early rheumatoid arthritis patients is maintained after discontinuation up to 1 year. Arthritis and Rheumatology.Conference: American College of Rheumatology/Association of Rheumatology Health Professionals Annual Scientific Meeting, ACR/ARHP 2015 San Francisco, CA United States. 6 ‐ 11 November 2015. 2015; Vol. 67.
-
- Atsumi T, Yamamoto K, Takeuchi T, Yamanaka H, Ishiguro N, Tanaka Y, et al. The first double‐blind, randomised, parallel‐group certolizumab pegol study in methotrexate‐naive early rheumatoid arthritis patients with poor prognostic factors, C‐OPERA, shows inhibition of radiographic progression. Annals of the Rheumatic Diseases 2016;75(1):75‐83. - PMC - PubMed
-
- Atsumi T, Yamamoto K, Takeuchi T, Yamanaka H, Ishiguro N, Tanaka Y, et al. The first early rheumatoid arthritis, certolizumab pegol, multicenter, double‐blind, randomized, parallel‐group study: C‐Opera, in patients fulfilling the 2010 ACR/EULAR classification criteria, demonstrates inhibition of joint damage progression. Annals of the Rheumatic Diseases.Conference: Annual European Congress of Rheumatology of the European League Against Rheumatism, EULAR 2014 Paris France. 11 ‐ 14 June 2014. 2014; Vol. 73.
-
- Atsumi T, Yamamoto K, Takeuchi T, Yamanaka H, Ishiguro N, Tanaka Y, et al. The first, multicenter, double‐blind, randomized, parallel‐group study of certolizumab pegol in early rheumatoid arthritis demonstrates inhibition of joint damage progression. Arthritis and Rheumatology.Conference: 2014 ACR/ARHP Annual Meeting Boston, MA United States. 14 ‐ 19 November 2014. 2014; Vol. 66:S1078‐9.
CDP870‐004 2001 {published and unpublished data}
-
- Emery P, Smolen J, Choy E, et al. CDP870 a novel, humanised tumour necrosis factor alpha inhibitor improves HRQOL. Late breaking abstract. European League Against Rheumatism Annual Conference. 2002.
-
- European Medicines Agency. Assesment report for Cimzia. Procedure No EMEA/H/C/001037. www.ema.europa.eu/docs/en_GB/document_library/EPAR_‐_Public_assessment_r.... London, 2009 (accessed 3rd July 2017):1‐47. [Procedure No.EMEA/H/C/001037]
-
- Keystone E, Choy E, Kalden J, Klareskog, Sany J, Smolen J, et al. CDP870, A novel pegylated, humanized TNF‐alpha inhibitor, is effective in treating the signs and symptoms of rheumatoid arthritis (RA). Abstract to Rheumatology annual scientific meeting [abstract # LB‐3]. 2001.
Choy 2002 {published data only}
-
- Choy EH, Hazleman B, Smith M, Moss K, Lisi L, Scott DG, et al. Efficacy of a novel pegylated humanized anti‐TNF fragment (CDP870) in patients with rheumatoid arthritis: a phase II double‐blinded, randomized, dose‐escalating trial. Rheumatology (Oxford) 2002;41(10):1133‐7. - PubMed
Choy 2012 {published data only}
-
- Choy E, McKenna F, Vencovsky J, Valente R, Goel N, Vanlunen B, et al. Certolizumab pegol plus MTX administered every 4 weeks is effective in patients with RA who are partial responders to MTX. Rheumatology (Oxford) 2012;51(7):1226‐34. - PubMed
-
- UCB. Clinical Study Summary Study No.: CDP870‐014. www.ucb.com/_up/ucb_com_patients/documents/C87032_CSS_20080608.pdf 2008 (accessed 3rd July 2017).
Emery 2015 {published data only}
-
- Emery P, Bingham C, Burmester GR, Bykerk VP, Furst D, Mariette X, et al. Improvements in workplace and household productivity following 52 weeks of treatment with certolizumab pegol in combination with methotrexate in DMARD‐naive patients with severe, active and progressive rheumatoid arthritis: Results from the c‐early randomized, double‐blind, controlled phase 3 study. Value in Health.Conference: ISPOR 18th Annual European Congress Milan, Italy.Conference. 7 ‐ 11 November 2015. 2015; Vol. 18:7.
-
- Emery P, Bingham CO, Burmester G‐R, Bykerk VP, Furst DE, Mariette X, et al. Improvements in patient‐reported outcomes and workplace and household productivity following 52 weeks of treatment with certolizumab pegol in combination with methotrexate in DMARD‐naive early rheumatoid arthritis patients: Results from the C‐early randomized, double‐blind, controlled phase 3 study. Annals of the Rheumatic Diseases.Conference: Annual European Congress of Rheumatology of the European League Against Rheumatism, EULAR 2015 Rome Italy. 10 ‐ 13 June 2015. 2015; Vol. 74:712‐3.
-
- Emery P, Bingham CO, Burmester G‐R, Bykerk VP, Furst DE, Mariette X, et al. SAT0165 Improvements in patient‐reported outcomes and workplace and household productivity following 52 weeks of treatment with certolizumab pegol in combination with methotrexate in dmard‐naïve early rheumatoid arthritis patients: results from the C‐Early randomized, double‐blind, controlled phase 3 study. Annals of the Rheumatic Diseases 2015;74(Suppl 2):712‐3.
-
- Emery P, Bingham CO, Burmester G‐R, Bykerk VP, Furst DE, Mariette X, et al. sSAT0164 The first study of certolizumab pegol in combination with methotrexate in dmard‐naïve early rheumatoid arthritis patients led to Sustained clinical response and inhibition of radiographic progression at 52 weeks: The C‐Early randomized, double‐blind, controlled phase 3 study. Annals of the Rheumatic Diseases 2015;74(Suppl 2):712.
-
- Weinblatt M, Bingham C, Burmester G, Bykerk V, Furst DE, Mariette X, et al. Early response as a predictor of long‐term remission in DMARD‐naive patients with severe, active and progressive rheumatoid arthritis treated with certolizumab pegol in combination with methotrexate. Arthritis and Rheumatology.Conference: American College of Rheumatology/Association of Rheumatology Health Professionals Annual Scientific Meeting, ACR/ARHP 2015 San Francisco, CA United States. 6 ‐ 11 November 2015. 2015; Vol. 67.
Fleischmann 2009 {published data only}
-
- Fleischmann R, Keininger DL, Tahiri‐Fitzgerald E, Mease P. Certolizumab pegol monotherapy 400mg every 4 weeks improves physical functioning and reduces pain in patients with rheumatoid arthritis Who have previously failed DMARD therapy. Program and abstracts of the European League Against Rheumatism (EULAR) Annual Meeting; Barcelona, Spain 13‐16 June [Abstract #0148]. 2007.
-
- Fleischmann R, Vencovsky J, Vollenhoven RF, Borenstein D, Box J, Coteur G, et al. Efficacy and safety of certolizumab pegol monotherapy every 4 weeks in patients with rheumatoid arthritis failing previous disease modifying antirheumatic therapy: the FAST4WARD study. Annals of the Rheumatic Diseases 2009;68(6):805‐11. - PMC - PubMed
-
- Keystone E, Mason D, Fleischmann R. Certolizumab pegol 400 mg every 4 weeks as monotherapy rapidly reduces disease activity in active rheumatoid arthritis. Program and abstracts of the American College of Rheumatology (ACR) 71st Annual Meeting; November 6 ‐ 11; Boston, Massachusetts. [Abstract #277]. 2007.
-
- Strand V, Brown M, Purcaru O, Richard L. Certolizumab pegol monotherapy improves productivity in patients with active rheumatoid arthritis: results from a phase III randomized controlled trial. Program and abstracts of the European League Against Rheumatism (EULAR) Annual Meeting; Barcelona, Spain 13 ‐ 16 June [Abstract #0478]. 2007.
Keystone 2008 {published data only}
-
- Curtis JR, Chen L, Luijtens K, Navarro‐Millan I, Goel N, Gervitz L, et al. Dose escalation of certolizumab pegol from 200 mg to 400 mg every other week provides no additional efficacy in rheumatoid arthritis: an analysis of individual patient‐level data. Arthritis and Rheumatism 2011;63(8):2203‐8. - PubMed
-
- Haraoui B, Bykerk VP, Vollenhoven R, Longueville M, Luijtens K, Ralston P, et al. Analysis of pooled data from two randomized controlled trials and their open‐label extensions: Long‐term safety in rheumatoid arthritis before and after certolizumab pegol dose increase/decrease. Arthritis and Rheumatology.Conference: 2014 ACR/ARHP Annual Meeting Boston, MA United States 14 ‐ 19 N0vember 2014. 2014; Vol. 66:S199.
-
- Keystone E, Heijde D, Mason D Jr, Landewé R, Vollenhoven RV, Combe B, et al. Certolizumab pegol plus methotrexate is significantly more effective than placebo plus methotrexate in active rheumatoid arthritis: findings of a fifty‐two‐week, phase III, multicenter, randomized, double‐blind, placebo‐controlled, parallel‐group study. Arthritis and Rheumatism 2008;58(11):3319‐29. - PubMed
-
- Keystone E, Landewé R, Vollenhoven R, Combe B, Strand V, Mease P, et al. 5‐Year results from the RAPID 1 trial and open‐label extension: long‐term safety and efficacy of certolizumab pegol in combination with methotrexate in the treatment of rheumatoid arthritis. Annals of the Rheumatic Diseases 2013;72(12):A228‐9. [10.1136/annrheumdis‐2013‐203695. [Epub ahead of print]] - PMC - PubMed
NCT00993317 {published data only}
-
- NCT00993317. A study of CDP870 as add‐on medication to methotrexate (MTX) in patients with rheumatoid arthritis. clinicaltrials.gov/ct2/show/study/NCT00993317 (firs treceived 9th October 2009).
Smolen 2009 {published data only}
-
- Haraoui B, Bykerk VP, Vollenhoven R, Longueville M, Luijtens K, Ralston P, et al. Analysis of pooled data from two randomized controlled trials and their open‐label extensions: Long‐term safety in rheumatoid arthritis before and after certolizumab pegol dose increase/decrease. Arthritis and Rheumatology.Conference: 2014 ACR/ARHP Annual Meeting Boston, MA United States 14 ‐ 19 N0vember 2014. 2014; Vol. 66:S199.
-
- Kavanaugh A, Smolen JS, Emery P, Purcaru O, Keystone E, Richard L, et al. Effect of certolizumab pegol with methotrexate on home and work place productivity and social activities in patients with active rheumatoid arthritis. Arthritis and Rheumatism 2009;61(11):1592‐600. - PubMed
-
- Landewé R, Strand V, Smolen J, Heijde D. Liquid formulation certolizumab pegol with methotrexate decreases progression of structural joint damage in rheumatoid arthritis patients: the RAPID 2 study. Program and abstracts of the American College of Rheumatology (ACR) 71st Annual Meeting; 6 ‐ 11 November 2007; Boston, Massachusetts. [Abstract #696]. 2007.
-
- Mease P, Mason D, Kavanaugh A, Smolen J. Efficacy and rapid response of certolizumab pegol liquid formulation in combination with methotrexate (MTX) in patients with active rheumatoid arthritis despite MTX therapy: results from the RAPID 2 study. Program and abstracts of the American College of Rheumatology (ACR) 71st Annual Meeting; 6 ‐ 11 November 2007; Boston, Massachusetts. [Abstract #941]. 2007.
-
- Schiff M, Keininger DL, Tahiri‐Fitzgerald E. Certolizumab pegol added onto methotrexate improves physical functioning and reduces pain in patients with rheumatoid arthritis who have an incomplete response to methotrexate: data from rapid 2. Program and abstracts of the European League Against Rheumatism (EULAR) Annual Meeting; Barcelona, Spain 13 ‐ 16 June [Abstract #0200]. 2007.
Smolen 2015 {published data only}
-
- NCT00674362. Rheumatoid arthritis (RA) moderate to low disease activity study (CERTAIN). clinicaltrials.gov/ct2/show/NCT00674362 (first received 5th May 2008).
-
- Smolen JS, Emery P, Ferraccioli G, Samborski W, Berenbaum F, Davies O, et al. Maintenance of remission in rheumatoid arthritis patients with low‐moderate disease activity following withdrawal of certolizumab PEGOL treatment: Week 52 results from the certain study. Annals of the Rheumatic Disease 2013;71 Suppl 3:361.
-
- Smolen JS, Emery P, Ferraccioli GF, Samborski W, Berenbaum F, Davies OR, et al. Certolizumab pegol in rheumatoid arthritis patients with low to moderate activity: the CERTAIN double‐blind, randomised, placebo‐controlled trial. Annals of the Rheumatic Diseases 2015; Vol. 74, issue 5:843‐50. [DOI: 10.1136/annrheumdis-2013-204632] - DOI - PMC - PubMed
-
- Smolen JS, Vollenhoven R, Kavanaugh A, Strand V, Vencovsky J, Schiff M, et al. Certolizumab pegol plus methotrexate 5‐year results from the rheumatoid arthritis prevention of structural damage (RAPID) 2 randomized controlled trial and long‐term extension in rheumatoid arthritis patients. Arthritis Research & Therapy 2015;17:245. - PMC - PubMed
Weinblatt 2012 {published data only}
-
- Weinblatt ME, Fleischmann R, Huizinga TW, Emery P, Pope J, Massarotti EM, et al. Efficacy and safety of certolizumab pegol in a broad population of patients with active rheumatoid arthritis: results from the REALISTIC phase IIIb study. Rheumatology (Oxford) 2012;51:2204‐14. - PubMed
-
- Weinblatt ME, Fleischmann R, Vollenhoven RF, Emery P, Huizinga TW, Cutolo M, et al. Twenty‐eight‐week results from the REALISTIC phase IIIb randomized trial: efficacy, safety and predictability of response to certolizumab pegol in a diverse rheumatoid arthritis population. Arthritis Research & Therapy 2015;17:325. [DOI: 10.1186/s13075-015-0841-9] - DOI - PMC - PubMed
Yamamoto (a) 2014 {unpublished data only}
-
- NCT00791921. Efficacy confirmation trial of CDP870 without coadministration of methotrexate (MTX) in Japanese rheumatoid arthritis (RA). clinicaltrials.gov/show/NCT00791921 (first received 14 November 2008).
-
- NCT00850343. Long‐term treatment study of certolizumab pegol without coadministration of methotrexate in Japanese rheumatoid arthritis (RA) patients. clinicaltrials.gov/ct2/show/NCT00850343?term=NCT00850343&rank=1 (first received 23rd February 2009).
-
- Takeuchi T, Yamamoto K, Yamanaka H, Ishiguro N, Tanaka Y, Eguchi K, et al. Better clinical responses seen early with the loading dose of certolizumab pegol are maintained until one year. Annals of the Rheumatic Diseases.Conference: Annual European Congress of Rheumatology of the European League Against Rheumatism, EULAR 2014 Paris France. 11 ‐ 14 June 2014. 2014; Vol. 73.
-
- Tanaka Y, Yamamoto K, Takeuchi T, Yamanaka H, Ishiguro N, Eguchi K, et al. Long‐term efficacy and safety of certolizumab pegol in Japanese rheumatoid arthritis patients who could not receive methotrexate: 52‐week results from an open‐label extension of the HIKARI study. Modern Rheumatology 2013; Vol. 24, issue 5:725‐33. [DOI: 10.3109/14397595.2013.865822] - DOI - PubMed
-
- Yamamoto, Kazuhiko, Takeuchi, Tsutomu, Yamanaka, Hisashi, et al. Efficacy and safety of certolizumab pegol without methotrexate co‐administration in Japanese patients with active rheumatoid arthritis: The HIKARI randomized, placebo‐controlled trial. Modern Rheumatology 2014;24:552‐560. - PubMed
Yamamoto (b) 2014 {published data only}
-
- NCT00791999. Efficacy confirmation trial of CDP870 as add‐on medication to methotrexate (MTX) in Japanese rheumatoid arthritis (RA). clinicaltrials.gov/ct2/show/study/NCT00791999?term=NCT00791999&rank=1 (first received 14th November 2008).
-
- Takeuchi T, Yamamoto K, Yamanaka H, Ishiguro N, Tanaka Y, Eguchi K, et al. Better clinical responses seen early with the loading dose of certolizumab pegol are maintained until one year. Annals of the Rheumatic Diseases.Conference: Annual European Congress of Rheumatology of the European League Against Rheumatism, EULAR 2014 Paris France. 11 ‐ 14 June 2014. 2014; Vol. 73.
-
- Tanaka Y, Yamamoto K, Takeuchi T, Yamanaka H, Ishiguro N, Eguchi K, et al. Long‐term efficacy and safety of certolizumab pegol in Japanese rheumatoid arthritis patients with an inadequate response to methotrexate: 52‐week results from an open‐label extension of the J‐RAPID study. Modern Rheumatology 2014;24(5):734‐43. - PMC - PubMed
-
- Yamamoto K, Takeuchi T, Yamanaka H, Ishiguro N, Tanaka Y, Eguchi K, et al. Efficacy and safety of certolizumab pegol plus methotrexate in Japanese rheumatoid arthritis patients with an inadequate response to methotrexate: the J‐RAPID randomized, placebo‐controlled trial. Modern Rheumatology 2014;24(5):715‐24. [DOI: 10.3109/14397595.2013.864224] - DOI - PubMed
-
- Yamanaka H, Yamamoto K, Takeuchi T, Ishiguro N, Tanaka Y, Eguchi K, et al. AB0468 Improved physical function, pain, and health related quality of life with certolizumab pegol in Japanese rheumatoid arthritis patients with an inadequate response to methotrexate: Results from the JRapid study. Annals of the Rheumatic Diseases 2013;71 Suppl 3:664. [DOI: 10.1136/annrheumdis-2012-eular.468] - DOI
Østergaard 2015 {published data only}
-
- Østergaard M, Axelsen MB, Jacobsson LTH, Schaufelberger C, Hansen MS, Bijlsma JWJ, et al. Dynamic magnetic resonance imaging in the assessment of the response to certolizumab pegol in rheumatoid arthritis patients: Results from a phase IIIB randomized study. Arthritis and Rheumatology.Conference: 2014 ACR/ARHP Annual Meeting Boston, MA United States.Conference Start: 20141114 Conference End: 20141119.Conference Publication: (var.pagings) 2014;66(pp S518‐S519):October.
-
- Østergaard M, Jacobsson LTH, Schaufelberger C, Hansen MS, Bijlsma JWJ, Dudek A, et al. MRI assessment of early response to certolizumab pegol in rheumatoid arthritis: a randomised, double‐blind, placebo‐controlled phase IIIb study applying MRI at weeks 0, 1, 2, 4, 8 and 16. Annals of the Rheumatic Diseases 2015;74(6):1156‐63. - PMC - PubMed
References to studies excluded from this review
Alten 2013 {published and unpublished data}
-
- Alten R, Fleischmann R, Vollenhoven R, Vencovsky J, Davies O, Stach C, et al. Long term tolerability and efficacy of a 4‐week‐administration of certolizumab pegol as monotherapy and combination therapy in rheumatoid arthritis; 5‐year‐data of an open extension study. Zeitschrift Für Rheumatologie 2013;72:111.
Bykerk 2015 {published data only}
-
- Bykerk VP, Bingham C, Burmester G, Furst DE, Mariette X, Purcaru O, et al. Reduction of disease burden on workplace and household productivity following 52 weeks of treatment with certolizumab pegol in combination with methotrexate in DMARD‐naive patients with active, severe, progressive rheumatoid arthritis. Arthritis and Rheumatology.Conference: American College of Rheumatology/Association of Rheumatology Health Professionals Annual Scientific Meeting, ACR/ARHP 2015 San Francisco, CA United States.Conference Start: 20151106 Conference End: 20151111.Confer 2015;67(no pagination):October.
Curtis 2014 {published data only}
-
- Curtis JR, Churchill M, Kivitz A, Samad A, Gauer L, Coteur G, et al. Randomization to patient‐reported RAPID3 versus physician‐based CDAI tools for prediction of treatment response and assessment of patient‐reported outcomes in rheumatoid arthritis patients receiving certolizumab pegol: Results from the predict study. Annals of the Rheumatic Diseases.Conference: Annual European Congress of Rheumatology of the European League Against Rheumatism, EULAR 2014 Paris France. 11 ‐14 June 2014. 2014; Vol. 73.
Curtis 2015a {published data only}
Curtis 2015b {published data only}
-
- Curtis JR, Longueville M, O'Brien C, Haraoui B. Improvement in disease activity and the long‐term risk of serious infectious events in rheumatoid arthritis patients treated with certolizumab pegol. Arthritis and Rheumatology.Conference: American College of Rheumatology/Association of Rheumatology Health Professionals Annual Scientific Meeting, ACR/ARHP 2015 San Francisco, CA United States. 6 ‐ 11 November 2015. 2015; Vol. 67.
Dose Flex 2007 {published data only}
-
- NCT00580840. Dosing flexibility study in patients with rheumatoid arthritis (DoseFlex). clinicaltrials.gov/ct2/show/results/NCT00580840 (first received 21st December 2007).
Fleischmann 2013 {published data only}
-
- Fleischmann R, Vollenhoven R, Vencovsky J, Alten R, Davies O, Stach C, et al. Long‐term safety and efficacy of 4‐weekly certolizumab pegol monotherapy and combination therapy in rheumatoid arthritis: 5‐year results from an open‐label extension study. Annals of the Rheumatic Diseases 2013;72:435.
Kavanaugh 2013 {published data only}
-
- Kavanaugh A, Smolen JS, Emery P, Keystone E, Strand V, Purcaru O, et al. Long‐term benefits over more than 4 years of certolizumab pegol combination therapy on workplace and household productivity, and participation in social activities in rheumatoid arthritis: Results from the open‐label extension study. Value in Health 2013;16:A570.
Kavanaugh 2014 {published data only}
-
- Kavanaugh A, Mease PJ, Strand V, Purcaru O, Curtis JR. PMS66 ‐ Effect of certolizumab pegol on workplace And household productivity In United States patients with rheumatoid arthritis with or without prior anti‐Tnf exposure: results from the Predict study. Value in Health 2014;17(3):A53.
Kivitz 2014 {published data only}
-
- Kivitz AJ, Schechtman J, Texter M, Fichtner A, Longueville M, Chartash EK. Vaccine responses in patients with rheumatoid arthritis treated with certolizumab pegol: results from a single‐blind randomized phase IV trial. Journal of Rheumatology 2014;41(4):648‐57. - PubMed
NCT00160641 {published data only}
-
- NCT00160641. A study of the safety and effectiveness of liquid certolizumab pegol in the treatment of signs and symptoms of rheumatoid arthritis and in prevention of joint damage in patients with active rheumatoid arthritis. clinicaltrials.gov/ct2/show/NCT00160641?term=NCT00160641&rank=1 (first received 8th September 2005).
NCT00160693 {published data only}
-
- NCT00160693. Open label long‐term safety study of certolizumab pegol (CZP) for patients with rheumatoid arthritis. clinicaltrials.gov/ct2/show/NCT00160693 (first received 6th September 2005).
NCT00753454 {published data only}
-
- NCT00753454. Open label extension for patients coming from the dosing flexibility study in patients with rheumatoid arthritis (RA) (Dose Flex II). clinicaltrials.gov/ct2/show/NCT00753454?term=NCT00753454&rank=1 (first received 5th June 2008).
NCT00843778 {published data only}
-
- NCT00843778. Follow‐up of rheumatoid arthritis (RA) moderate to low disease activity study (CERTAIN 2). clinicaltrials.gov/ct2/show/NCT00843778?term=NCT00843778&rank=1 (first received 5th January 2009).
NCT00851318 {published data only}
-
- NCT00851318. Long‐term treatment study of certolizumab pegol (CDP870) as add‐on medication to methotrexate in Japanese rheumatoid arthritis (RA) Patients. clinicaltrials.gov/ct2/show/NCT00851318?term=certolizumab+and+arthritis&... (first received 23rd February 2009).
NCT00993668 {unpublished data only}
-
- NCT00993668. Assessing the use of certolizumab pegol in adult subjects with rheumatoid arthritis on the antibody response when receiving influenza virus and pneumococcal vaccines. clinicaltrials.gov/ct2/show/results/NCT00993668?term=NCT00993668&rank=1 (first received 9th October 2009).
NCT01197066 {published data only}
-
- NCT01197066. Open‐label, extension study of CDP870 in patients with rheumatoid arthritis. clinicaltrials.gov/ct2/show/NCT01197066? (first received 17th March 2010).
NCT01255761 PREDICT {published data only}
-
- NCT01255761. A comparison of two assessment tools in predicting treatment success of cimzia in rheumatoid arthritis subjects (PREDICT). clinicaltrials.gov/ct2/show/NCT01255761?term=NCT01255761&rank=1 (first received 6th December 2010).
NCT01292265 {published data only}
-
- NCT01292265. A 12 week study to assess changes in joint inflammation using ultrasonography in patients with rheumatoid arthritis (RA). clinicaltrials.gov/ct2/show/NCT01292265?term=certolizumab+and+arthritis&... (first received 7th February 2011).
NCT01374971 {published data only}
-
- NCT01374971. Rheumatoid arthritis treatment and biopsy study assessing certolizumab pegol (Cimzia). clinicaltrials.gov/ct2/show/results/NCT01374971?term=NCT01374971&rank=1 (first received 14th June 2011).
NCT01443364 {published data only}
-
- NCT01443364. Open label study to assess the predictability of early response to certolizumab pegol in patients with rheumatoid arthritis (SPEED). clinicaltrials.gov/ct2/show/NCT01443364?term=certolizumab+and+arthritis&... (first received 27th September 2011).
NCT01526434 {published data only}
-
- NCT01526434. Health‐related quality of life and patient‐reported outcomes in rheumatoid arthritis patients treated with certolizumab pegol. clinicaltrials.gov/ct2/show/NCT01526434?term=certolizumab+and+arthritis&... (first received 1st February 2012).
NCT02319642 {published data only}
-
- NCT02319642. An open‐label extension study of certolizumab pegol in Chinese patients with rheumatoid arthritis who enrolled in RA0044 (RAPID‐C OLE). clinicaltrials.gov/ct2/show/NCT02319642?term=certolizumab+and+arthritis&... (first received 15th December 2014).
NCT02586246 {published data only}
-
- NCT02586246. Long‐term treatment study of CDP870 self‐injection in patients with active rheumatoid arthritis who are participating in the long‐term treatment studies (Study 275‐08‐002 or Study 275‐08‐004) of CDP870. clinicaltrials.gov/ct2/show/NCT02586246?term=certolizumab+and+arthritis&... (first received 23rd October 2015).
References to ongoing studies
NCT01295151 {published data only}
-
- NCT01295151. SWITCH clinical trial for patients with rheumatoid arthritis who have failed an initial TNF‐blocking drug (SWITCH). clinicaltrials.gov/ct2/show/NCT01295151 (first received 11th February 2011).
-
- Navarro C, Nuria C, Brown S, Bosworth A, et al. The 'Switch’ study protocol: a randomised‐controlled trial of switching to an alternative tumour‐necrosis factor (TNF)‐inhibitor drug or abatacept or rituximab in patients with rheumatoid arthritis who have failed an initial TNF‐inhibitor drug. BMC Musculoskeletal Disorders 2014; Vol. 15:452. [DOI: 10.1186/1471-2474-15-452] - DOI - PMC - PubMed
NCT01489384 {published data only}
-
- NCT01489384. Cimzia treatment in rheumatoid arthritis: randomizing to stop versus continue disease‐modifying anti‐rheumatic drug(s). clinicaltrials.gov/ct2/show/NCT01489384?term=certolizumab+and+arthritis&... (first received 7th December 2011).
NCT01491815 {published data only}
-
- NCT01491815. Active conventional therapy compared to three different biologic treatments in early rheumatoid arthritis with subsequent dose reduction. clinicaltrials.gov/ct2/show/NCT01491815?term=certolizumab+and+arthritis&... (first received 8th December 2011).
NCT01500278 {published data only}
-
- NCT01500278. Study to assess the short‐ and long‐term efficacy of certolizumab pegol plus methotrexate compared to adalimumab plus methotrexate in subjects with moderate to severe rheumatoid arthritis (RA) inadequately responding to methotrexate. clinicaltrials.gov/ct2/show/NCT01500278?term=certolizumab+and+arthritis&... (first received 22nd December 2011).
NCT01602302 {published data only}
-
- NCT01602302. Ultrasound and withdrawal of biological DMARDs in rheumatoid arthritis. clinicaltrials.gov/ct2/show/NCT01602302?term=certolizumab+and+arthritis&... (first received 16th May 2012).
NCT02151851 {published data only}
-
- NCT02151851. A study of certolizumab pegol as additional therapy in Chinese patients with active rheumatoid arthritis (RAPID‐C). clinicaltrials.gov/ct2/show/NCT02151851?term=certolizumab+and+arthritis&... (first received 28th May 2014).
NCT02293590 {published data only}
-
- NCT02293590. RICE: Remission by Intra‐articular Injection Plus Certolizumab. clinicaltrials.gov/ct2/show/NCT02293590?term=certolizumab+and+arthritis&... (first received 30th October 2014).
NCT02430909 {published data only}
-
- NCT02430909. Multiple dose study of UCB4940 as add‐on to certolizumab pegol in subjects with rheumatoid arthritis. clinicaltrials.gov/ct2/show/NCT02430909?term=certolizumab+and+arthritis&... (first received 27th April 2015).
NCT02466581 {published data only}
-
- NCT02466581. Dose reduction for early rheumatoid arthritis patients with low disease activity. clinicaltrials.gov/ct2/show/NCT02466581?term=certolizumab+and+arthritis&... (first received 29th May 2015).
Additional references
Abasolo 2016
-
- Abasolo L, Ivorra‐Cortes J, Leon L, Jover JA, Fernandez‐Gutierrez B, Rodriguez‐Rodriguez L. Influence of demographic and clinical factors on the mortality rate of a rheumatoid arthritis cohort: A 20‐year survival study. Seminars in Arthritis and Rheumatism 2016;45(5):533‐8. - PubMed
Arnett 1988
-
- Arnett FC, Edworthy SM, Bloch DA, McShane DJ, Fries JF, Cooper NS, et al. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis and Rheumatism 1988;31(3):315‐24. - PubMed
Bhandari 2004
Blumenauer 2002
Bongartz 2006
-
- Bongartz T, Sutton AJ, Sweeting MJ, Buchan I, Matteson EL, Montori V. Anti‐TNF antibody therapy in rheumatoid arthritis and the risk of serious infections and malignancies: systematic review and meta‐analysis of rare harmful effects in randomized controlled trials. JAMA 2006;295(19):2275‐85. - PubMed
Brennan 2008
Bykerk 2013
-
- Bykerk VP, Cush J, Winthrop K, Calabrese L, Lortholary O, Longueville M, et al. Update on the safety profile of certolizumab pegol in rheumatoid arthritis: an integrated analysis from clinical trials. Annals of the Rheumatic Diseases 2015;74(1):96‐103. [DOI: 10.1136/annrheumdis-2013-203660] - DOI - PMC - PubMed
Chen 2006
-
- Chen YF, Jobanputra P, Barton P, Jowett S, Bryan S, Clark W, et al. A systematic review of the effectiveness of adalimumab, etanercept and infliximab for the treatment of rheumatoid arthritis in adults and an economic evaluation of their cost‐effectiveness. Health Technology Assessment 2006; Vol. 10, issue 42:1‐235. - PubMed
Cross 2014
-
- Cross M, Smith E, Hoy D, Carmona L, Wolfe F, Vos T, et al. The global burden of rheumatoid arthritis: estimates from the global burden of disease 2010 study. Annals of the Rheumatic Diseases 2014;73(7):1316‐22. - PubMed
Doran 2002
-
- Doran MF, Pond GR, Crowson CS, O'Fallon WM, Gabriel SE. Trends in incidence and mortality in rheumatoid arthritis in Rochester, Minnesota, over a forty‐year period. Arthritis and Rheumatism 2002;46:625‐31. - PubMed
Dörner 2016
EMA 2009
-
- European Medical Agency. Assesment report for Cimzia. Procedure No EMEA/H/C/001037. www.ema.europa.eu/docs/en_GB/document_library/EPAR_‐_Public_assessment_r.... London, 2009 (accessed 4th July 2017):1‐47. [Procedure No.EMEA/H/C/001037]
Emparanza 2015
-
- Emparanza JI, Cabello JB, Burls AJE. Does evidence‐based practice improve patient outcomes? An analysis of a natural experiment in a Spanish hospital. Journal of Evaluation in Clinical Practice 2015;21(6):1059‐65. - PubMed
FDA 2013
-
- Center for Drug Evaluation and Research. Postmarket Drug Safety Information for Patients and Providers ‐ Information for Healthcare Professionals: Cimzia (certolizumab pegol), Enbrel (etanercept), Humira (adalimumab), and Remicade (infliximab). www.fda.gov/drugs/drugsafety/postmarketdrugsafetyinformationforpatientsa... (accessed 7 Jul 2014).
Felson 2011
Glanville 2006
Hazes 2010
-
- Hazes JM, Taylor P, Strand V, Purcaru O, Coteur G, Mease P. Physical function improvements and relief from fatigue and pain are associated with increased productivity at work and at home in rheumatoid arthritis patients treated with certolizumab pegol. Rheumatology (Oxford) 2010;49(10):1900‐10. - PMC - PubMed
Higgins 2011
-
- Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from www.handbook.cochrane.org.
Jobanputra 2012
-
- Jobanputra P, Maggs F, Deeming A, Carruthers D, Rankin E, Jordan AC, et al. A randomised efficacy and discontinuation study of etanercept versus adalimumab (RED SEA) for rheumatoid arthritis: a pragmatic, unblinded, non inferiority study of first TNF inhibitor use: outcomes over 2 years. BMJ Open 2012;2(6):e001395. - PMC - PubMed
Keystone 2012
-
- Keystone EC, Combe B, Smolen J, Strand V, Goel N, Vollenhoven R, et al. Sustained efficacy of certolizumab pegol added to methotrexate in the treatment of rheumatoid arthritis: 2‐year results from the RAPID 1 trial. Rheumatology (Oxford) 2012;51(9):1628‐38. - PubMed
Kharlamova 2016
Laird 1990
-
- Laird NM, Wang F. Estimating rates of change in randomized clinical trials. Controlled Clinical Trials 1990;11(6):405‐19. - PubMed
Laupacis 1988
-
- Laupacis A, Sackett DL, Roberts RS. An assessment of clinically useful measures of the consequences of treatment. New England Journal of Medicine 1988;318(26):1728‐33. - PubMed
Lethaby 2013
Lopez‐Olivo 2014
Lundberg 2013
-
- Lundberg K, Bengtsson C, Kharlamova N, Reed E, Jiang X, Kallberg H, et al. Genetic and environmental determinants for disease risk in subsets of rheumatoid arthritis defined by the anticitrullinated protein/peptide antibody fine specificity profile. Annals of the Rheumatic Diseases 2013;72(5):652‐8. - PubMed
MacGregor 2000
-
- MacGregor AJ, Snieder H, Rigby AS, Koskenvuo M, Kaprio J, Aho K, et al. Characterizing the quantitative genetic contribution to rheumatoid arthritis using data from twins. Arthritis and Rheumatism 2000;43(1):30‐7. - PubMed
Meune 2009
-
- Meune C, Touze E, Trinquart L, Allanmore Y. Trends in cardiovascular mortality in patients with rheumatoid arthritis over 50 years: a systematic review and meta‐analysis of cohort studies. Rheumatology 2009;48:1309‐13. - PubMed
Navarro‐Millán 2013
Navarro‐Sarabia 2005
NICE 2009
-
- UCB. Certolizumab pegol (CIMZIA©) for the treatment of rheumatoid arthritis. Single technology appraisal (STA) manufacturer submission to NICE. NICE 2009:1‐180.
Okada 2014
Puolakka 2005
-
- Puolakka K, Kautiainen H, Möttönen T, Hannonen P, Korpela M, Hakala M, et al. FIN‐RACo Trial Group. Early suppression of disease activity is essential for maintenance of work capacity in patients with recent‐onset rheumatoid arthritis: five‐year experience from the FIN‐RACo trial. Arthritis and Rheumatology 2005;52(1):36‐4. - PubMed
Roubille 2015
-
- Roubille C, Richer V, Starnino T, McCourt C, McFarlane A, Fleming P, et al. The effects of tumour necrosis factor inhibitors, methotrexate, non‐steroidal anti‐inflammatory drugs and corticosteroids on cardiovascular events in rheumatoid arthritis, psoriasis and psoriatic arthritis: a systematic review and meta‐analysis. Annals of the Rheumatic Diseases 2015;74(3):480‐9. - PMC - PubMed
Singh 2009
Singh 2010
Singh 2011
Singh 2012
-
- Singh JA, Furst DE, Bharat A, Curtis JR, Kavanaugh AF, Kremer JM, et al. 2012 update of the 2008 American College of Rheumatology recommendations for the use of disease‐modifying antirheumatic drugs and biologic agents in the treatment of rheumatoid arthritis. Arthritis Care and Research 2012;64:625‐39. - PMC - PubMed
Singh 2015
Singh 2016
-
- Singh A, Saag KG, Bridges SL, Akl A, Bannuru RR, Sullivan MC, et al. 2015 American College of Rheumatology guideline for the treatment of rheumatoid arthritis. Arthritis Care & Research 2016;68(1):1‐25. - PubMed
Smolen 2014
Sokka 2003
-
- Sokka T, Krishnan E, Hakkinen A, Hannonen P. Functional disability in rheumatoid arthritis patients compared with a community population in Finland. Arthritis and Rheumatism 2003;48(1):59‐63. [PUBMED: 12528104] - PubMed
Thompson 1999
-
- Thompson SG, Sharp SJ. Explaining heterogeneity in meta‐analysis: a comparison of methods. Statistics in Medicine 1999;18(20):2693‐708. - PubMed
Tureson 2013
-
- Turesson C. Extra‐articular rheumatoid arthritis. Current Opinion in Rheumatology 2013;23(3):360–6. - PubMed
Wong 2006a
Wong 2006b
-
- Wong SS, Wilczynski NL, Haynes RB. Optimal CINAHL search strategies for identifying therapy studies and review articles. Journal of Nursing Scholarship 2006;38(2):194‐9. - PubMed
Yee 2003
-
- Yee CS, Filer A, Pace A, Douglas K, Situnayake D, Rowe IF. West Midlands Rheumatology Services and Training Committee. The prevalence of patients with rheumatoid arthritis in the West Midlands fulfilling the BSR criteria for anti‐tumour necrosis factor therapy: an out‐patient study. Rheumatology (Oxford) 2003;42:856‐9. - PubMed
Young 2000
-
- Young A, Dixey J, Cox N, Davies P, Devlin J, Emery P, et al. How does functional disability in early rheumatoid arthritis affect patients and their lives? Results of 5 years of follow‐up in 732 patients from the early RA study (ERAS). Rheumatology 2000;39(6):603‐11. - PubMed
Yusuf 1991
-
- Yusuf S, Wittes J, Probstfield J, Tyroler HA. Analysis and interpretation of treatment effects in subgroups of patients in randomized clinical trials. JAMA 1991;266(1):93‐8. - PubMed
Zhou 2014
-
- Zhou Q, Zhou Y, Chen H, Wang Z, Tang Z, Liu J. The efficacy and safety of certolizumab pegol (CZP) in the treatment of active rheumatoid arthritis (RA): a meta‐analysis from nine randomized controlled trials. International Journal of Clinical and Experimental Medicine 2014;7(11):3870‐80. - PMC - PubMed
References to other published versions of this review
Ruiz Garcia 2009
Ruiz Garcia 2011
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

