A Small Molecule Causes a Population Shift in the Conformational Landscape of an Intrinsically Disordered Protein
- PMID: 28885015
- PMCID: PMC5962290
- DOI: 10.1021/jacs.7b01380
A Small Molecule Causes a Population Shift in the Conformational Landscape of an Intrinsically Disordered Protein
Abstract
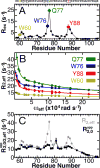
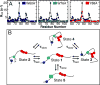
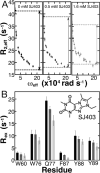
Intrinsically disordered proteins (IDPs) have roles in myriad biological processes and numerous human diseases. However, kinetic and amplitude information regarding their ground-state conformational fluctuations has remained elusive. We demonstrate using nuclear magnetic resonance (NMR)-based relaxation dispersion that the D2 domain of p27Kip1, a prototypical IDP, samples multiple discrete, rapidly exchanging conformational states. By combining NMR with mutagenesis and small-angle X-ray scattering (SAXS), we show that these states involve aromatic residue clustering through long-range hydrophobic interactions. Theoretical studies have proposed that small molecules bind promiscuously to IDPs, causing expansion of their conformational landscapes. However, on the basis of previous NMR-based screening results, we show here that compound binding only shifts the populations of states that existed within the ground state of apo p27-D2 without changing the barriers between states. Our results provide atomic resolution insight into how a small molecule binds an IDP and emphasize the need to examine motions on the low microsecond time scale when probing these types of interactions.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

