Targeting the central projection of the dural trigeminovascular system for migraine prophylaxis
- PMID: 28885085
- PMCID: PMC6446423
- DOI: 10.1177/0271678X17729280
Targeting the central projection of the dural trigeminovascular system for migraine prophylaxis
Abstract
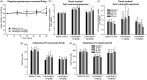
Migraine abortives likely target both peripheral-dural and central trigeminovascular mechanisms in mediating their therapeutic effects. However, in preclinical assays, many migraine preventives have little success at inhibiting similar trigeminovascular-mediated peripheral changes within the dural microenvironment. In addition, their effects on central trigeminovascular neuronal responses are largely unknown. Using a validated preclinical model of acute dural-intracranial (migraine-like) head pain, using Sprague Dawley rats, we tested whether migraine preventives suppress ongoing firing of central trigeminocervical neurons, and evoked responses to cranial neurovascular activation. Flunarizine, sodium valproate, propranolol, and amitriptyline, all dose-dependently inhibited ongoing spontaneous firing of dural trigeminovascular neurons, and differentially affected neuronal responses to intracranial-dural and extracranial-cutaneous somatosensory stimulation. Lamotrigine, only effective in the treatment of migraine aura, did not affect responses. These data provide a mechanistic rationale for the clinical effects of migraine preventives in the treatment of migraine, via the modulation of dural-responsive central trigeminovascular neurons. Also, given their limited effect on peripheral dural vasdilatory responses, these data also suggest that migraine preventives specifically target central, rather than peripheral, components of trigeminal neurovascular mechanisms involved in migraine pathophysiology, to mediate their preventive action. Finally, these data further validate this preclinical model of central trigeminovascular activation to screen migraine preventives.
Keywords: Migraine; central; peripheral; preventives; trigeminovascular system.
Figures





References
-
- Noseda R, Burstein R. Migraine pathophysiology: anatomy of the trigeminovascular pathway and associated neurological symptoms, cortical spreading depression, sensitization, and modulation of pain. Pain 2013; 154(Suppl 1): S44–S53. - PubMed
-
- Burstein R, Yarnitsky D, Goor-Aryeh I, et al. An association between migraine and cutaneous allodynia. Ann Neurol 2000; 47: 614–624. - PubMed
-
- Penfield W, McNaughton F. Dural headache and innervation of the dura mater. Arch Neurol Psychiatry 1940; 44: 43–75.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

