Anti-HIV Activities and Mechanism of 12-O-Tricosanoylphorbol-20-acetate, a Novel Phorbol Ester from Ostodes katharinae
- PMID: 28885587
- PMCID: PMC6151696
- DOI: 10.3390/molecules22091498
Anti-HIV Activities and Mechanism of 12-O-Tricosanoylphorbol-20-acetate, a Novel Phorbol Ester from Ostodes katharinae
Abstract
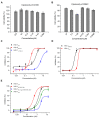
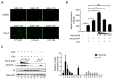
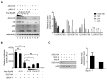
APOBEC3G is a member of the human cytidine deaminase family that restricts Vif-deficient viruses by being packaged with progeny virions and inducing the G to A mutation during the synthesis of HIV-1 viral DNA when the progeny virus infects new cells. HIV-1 Vif protein resists the activity of A3G by mediating A3G degradation. Phorbol esters are plant-derived organic compounds belonging to the tigliane family of diterpenes and could activate the PKC pathway. In this study, we identified an inhibitor 12-O-tricosanoylphorbol-20-acetate (hop-8), a novel ester of phorbol which was isolated from Ostodes katharinae of the family Euphorbiaceae, that inhibited the replication of wild-type HIV-1 and HIV-2 strains and drug-resistant strains broadly both in C8166 cells and PBMCs with low cytotoxicity and the EC50 values ranged from 0.106 μM to 7.987 μM. One of the main mechanisms of hop-8 is to stimulate A3G expressing in HIV-1 producing cells and upregulate the A3G level in progeny virions, which results in reducing the infectivity of the progeny virus. This novel mechanism of hop-8 inhibition of HIV replication might represents a promising approach for developing new therapeutics for HIV infection.
Keywords: 12-O-tricosanoylphorbol-20-acetate; APOBEC3G; HIV; Vif; antiviral agent; phorbol ester.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
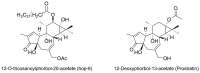

Similar articles
-
Identification of a novel HIV-1 inhibitor targeting Vif-dependent degradation of human APOBEC3G protein.J Biol Chem. 2015 Apr 17;290(16):10504-17. doi: 10.1074/jbc.M114.626903. Epub 2015 Feb 27. J Biol Chem. 2015. PMID: 25724652 Free PMC article.
-
Vpr14-88-Apobec3G fusion protein is efficiently incorporated into Vif-positive HIV-1 particles and inhibits viral infection.PLoS One. 2008 Apr 16;3(4):e1995. doi: 10.1371/journal.pone.0001995. PLoS One. 2008. PMID: 18414671 Free PMC article.
-
Various strategies for developing APOBEC3G protectors to circumvent human immunodeficiency virus type 1.Eur J Med Chem. 2023 Mar 15;250:115188. doi: 10.1016/j.ejmech.2023.115188. Epub 2023 Feb 6. Eur J Med Chem. 2023. PMID: 36773550 Review.
-
An analog of camptothecin inactive against Topoisomerase I is broadly neutralizing of HIV-1 through inhibition of Vif-dependent APOBEC3G degradation.Antiviral Res. 2016 Dec;136:51-59. doi: 10.1016/j.antiviral.2016.11.001. Epub 2016 Nov 5. Antiviral Res. 2016. PMID: 27825797 Free PMC article.
-
Apobec3G-Based Strategies to Defeat HIV Infection.Curr HIV Res. 2016;14(3):217-24. doi: 10.2174/1570162x14999160224100541. Curr HIV Res. 2016. PMID: 26957196 Review.
Cited by
-
Bromodomain and Extra-Terminal Inhibitor BMS-986158 Reverses Latent HIV-1 Infection In Vitro and Ex Vivo by Increasing CDK9 Phosphorylation and Recruitment.Pharmaceuticals (Basel). 2022 Mar 10;15(3):338. doi: 10.3390/ph15030338. Pharmaceuticals (Basel). 2022. PMID: 35337136 Free PMC article.
-
The Neuropeptides Vasoactive Intestinal Peptide and Pituitary Adenylate Cyclase-Activating Polypeptide Control HIV-1 Infection in Macrophages Through Activation of Protein Kinases A and C.Front Immunol. 2018 Jun 12;9:1336. doi: 10.3389/fimmu.2018.01336. eCollection 2018. Front Immunol. 2018. PMID: 29951068 Free PMC article.
-
Plant-Based Natural Products and Extracts: Potential Source to Develop New Antiviral Drug Candidates.Molecules. 2021 Oct 14;26(20):6197. doi: 10.3390/molecules26206197. Molecules. 2021. PMID: 34684782 Free PMC article. Review.
-
Aromatic disulfides as potential inhibitors against interaction between deaminase APOBEC3G and HIV infectivity factor.Acta Biochim Biophys Sin (Shanghai). 2022 May 25;54(5):725-735. doi: 10.3724/abbs.2022049. Acta Biochim Biophys Sin (Shanghai). 2022. PMID: 35920198 Free PMC article.
-
Anti-HIV Activity of Tigliane Derivatives from Euphorbia nicaeensis Roots.Molecules. 2025 Mar 25;30(7):1452. doi: 10.3390/molecules30071452. Molecules. 2025. PMID: 40286058 Free PMC article.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

