An overview of the clinical applications of optical coherence tomography angiography
- PMID: 28885606
- PMCID: PMC5811700
- DOI: 10.1038/eye.2017.181
An overview of the clinical applications of optical coherence tomography angiography
Abstract
Optical coherence tomography angiography (OCTA) has emerged as a novel, non-invasive imaging modality that allows the detailed study of flow within the vascular structures of the eye. Compared to conventional dye angiography, OCTA can produce more detailed, higher resolution images of the vasculature without the added risk of dye injection. In our review, we discuss the advantages and disadvantages of this new technology in comparison to conventional dye angiography. We provide an overview of the current OCTA technology available, compare the various commercial OCTA machines technical specifications and discuss some future software improvements. An approach to the interpretation of OCTA images by correlating images to other multimodal imaging with attention to identifying potential artefacts will be outlined and may be useful to ophthalmologists, particularly those who are currently still unfamiliar with this new technology. This review is based on a search of peer-reviewed published papers relevant to OCTA according to our current knowledge, up to January 2017, available on the PubMed database. Currently, many of the published studies have focused on OCTA imaging of the retina, in particular, the use of OCTA in the diagnosis and management of common retinal diseases such as age-related macular degeneration and retinal vascular diseases. In addition, we describe clinical applications for OCTA imaging in inflammatory diseases, optic nerve diseases and anterior segment diseases. This review is based on both the current literature and the clinical experience of our individual authors, with an emphasis on the clinical applications of this imaging technology.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
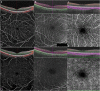

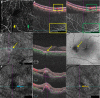
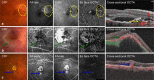

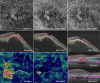

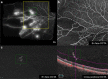
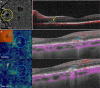


References
-
- Tan AC, Dansingani KK, Yannuzzi LA, Sarraf D, Freund KB. Type 3 Neovascularization imaged with cross-sectional and en face optical coherence tomography angiography. Retina 2017; 37(2): 234–246. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

