Historical time to disease progression and progression-free survival in patients with recurrent/refractory neuroblastoma treated in the modern era on Children's Oncology Group early-phase trials
- PMID: 28885700
- PMCID: PMC5716896
- DOI: 10.1002/cncr.30934
Historical time to disease progression and progression-free survival in patients with recurrent/refractory neuroblastoma treated in the modern era on Children's Oncology Group early-phase trials
Abstract
Background: Early-phase trials in patients with recurrent neuroblastoma historically used an objective "response" of measureable disease (Response Evaluation Criteria In Solid Tumors [RECIST], without bone/bone marrow assessment) to select agents for further study. Historical cohorts may be small and potentially biased; to the authors' knowledge, disease recurrence studies from international registries are outdated. Using a large recent cohort of patients with recurrent/refractory neuroblastoma from Children's Oncology Group (COG) modern-era early-phase trials, the authors determined outcome and quantified parameters for designing future studies.
Methods: The first early-phase COG trial enrollment (sequential) of 383 distinct patients with recurrent/refractory neuroblastoma on 23 phase 1, 3 phase 1/2, and 9 phase 2 trials (August 2002 to January 2014) was analyzed for progression-free survival (PFS), overall survival (OS), and time to disease progression (TTP). Planned frontline therapy for patients with high-risk neuroblastoma included hematopoietic stem cell transplantation (approximately two-thirds of patients underwent ≥1 hematopoietic stem cell transplantation); 13.2% of patients received dinutuximab.
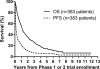
Results: From the time of the patient's first early-phase trial enrollment (383 patients), the 1-year and 4-year PFS rates ( ± standard error) were 21% ± 2% and 6% ± 1%, respectively, whereas the 1-year and 4-year OS rates were 57% ± 3% and 20% ± 2%, respectively. The median TTP was 58 days (interquartile range, 31-183 days [350 patients]); the median follow-up was 25.3 months (33 patients were found to be without disease recurrence/progression). The median time from diagnosis to first disease recurrence/progression was 18.7 months (range, 1.4-64.8 months) (176 patients). MYCN amplification and 11q loss of heterozygosity were prognostic of worse PFS and OS (P = .003 and P<.0001, respectively, and P = .02 and P = .03, respectively) after early-phase trial enrollment.
Conclusions: This recent COG cohort of patients with recurrent/refractory neuroblastoma is inclusive and representative. To the authors' knowledge, the current study is the first meta-analysis of PFS, TTP, and OS within the context of modern therapy. These results will inform the design of future phase 2 studies by providing a) historical context during the search for more effective agents; and, b) factors prognostic of PFS and OS after disease recurrence to stratify randomization. Cancer 2017;123:4914-23. © 2017 American Cancer Society.
Keywords: International Neuroblastoma Response Criteria (INRC); Response Evaluation Criteria In Solid Tumors (RECIST); endpoints; historical standard; phase 2 design; prognostic.
© 2017 American Cancer Society.
Conflict of interest statement
All authors (WL, RB, BW, EF, DG, CVR, AN, and JP) declare that they have no conflicts of interest.
Figures







References
-
- Maris JM, Hogarty MD, Bagatell R, et al. Neuroblastoma. Lancet. 2007;369:2106–2120. - PubMed
-
- Brodeur GM. Neuroblastoma: biological insights into a clinical enigma. Nat Rev Cancer. 2003;3:203–216. - PubMed
-
- Therasse P, Arbuck SG, Eisenhauer EA, et al. New guidelines to evaluate the response to treatment in solid tumors. J National Cancer Inst. 2000 Feb 2;92(3):205–16. - PubMed
-
- Brodeur GM, Pritchard J, Berthold F, et al. Revisions of the international criteria for neuroblastoma diagnosis, staging, and response to treatment. J Clin Oncol. 1993 Aug;11(8):1466–1477. - PubMed
-
- Castel V, Garcia-Miguel P, Canete A, et al. Prospective evaluation of the International Neuroblastoma Staging System (INSS) and the International Neuroblastoma Response Criteria (INRC) in a multicentre setting. Eur J Cancer. 1999 Apr;35(4):606–611. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

