Balancing anti-inflammatory and anti-oxidant responses in murine bone marrow derived macrophages
- PMID: 28886148
- PMCID: PMC5590945
- DOI: 10.1371/journal.pone.0184469
Balancing anti-inflammatory and anti-oxidant responses in murine bone marrow derived macrophages
Abstract
Rationale: The underlying pathophysiology of bronchopulmonary dysplasia includes a macrophage-mediated host response orchestrated by anti-inflammatory peroxisome proliferator-activated receptor gamma (PPARγ) and anti-oxidant nuclear factor (erythroid-derived 2)-like 2 (Nrf2). These have not yet been studied in combination. This study tested the hypothesis that combined inflammatory and oxidative stressors would interact and change PPARγ- and Nrf2-regulated gene expression and antioxidant capacity. Therefore, we investigated the effect of dual stimulation with lipopolysaccharide and hyperoxia in murine bone marrow-derived macrophages (BMDM).
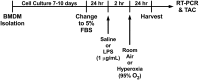
Methods: Sub-confluent BMDM from wild-type C57BL/6J mice were treated with lipopolysaccharide (LPS) 1ug/mL for 2 hours followed by room air (21% oxygen) or hyperoxia (95% oxygen) for 24 hours. Taqman real time-polymerase chain reaction gene expression assays, total antioxidant capacity assays, and Luminex assays were performed.
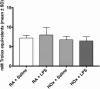
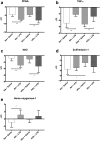
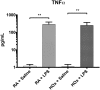
Results: Supernatants of cultured BMDM contained significant antioxidant capacity. In room air, LPS treatment decreased expression of PPARγ and Nrf2, and increased expression of tumor necrosis factor-alpha and heme oxygenase-1; similar findings were observed under hyperoxic conditions. LPS treatment decreased cellular total antioxidant capacity in room air but not in hyperoxia. Increased expression of sulfiredoxin-1 in response to hyperoxia was not observed in LPS-treated cells. Dual stimulation with LPS treatment and exposure to hyperoxia did not have synergistic effects on gene expression. Cellular total antioxidant capacity was not changed by hyperoxia exposure.
Conclusions: Our hypothesis was supported and we demonstrate an interaction between inflammatory and oxidative stressors in a model system of bronchopulmonary dysplasia pathogenesis. The protective anti-oxidant effect of cell culture media may have protected the cells from the most deleterious effects of hyperoxia.
Conflict of interest statement
Figures
Similar articles
-
Induction of Nrf2-mediated genes by Antrodia salmonea inhibits ROS generation and inflammatory effects in lipopolysaccharide-stimulated RAW264.7 macrophages.Food Funct. 2015 Jan;6(1):230-41. doi: 10.1039/c4fo00869c. Epub 2014 Nov 7. Food Funct. 2015. PMID: 25380370
-
Antioxidant signaling via Nrf2 counteracts lipopolysaccharide-mediated inflammatory responses in foam cell macrophages.Free Radic Biol Med. 2011 May 15;50(10):1382-91. doi: 10.1016/j.freeradbiomed.2011.02.036. Epub 2011 Mar 5. Free Radic Biol Med. 2011. PMID: 21382476
-
Astaxanthin exerts anti-inflammatory and antioxidant effects in macrophages in NRF2-dependent and independent manners.J Nutr Biochem. 2018 Dec;62:202-209. doi: 10.1016/j.jnutbio.2018.09.005. Epub 2018 Sep 19. J Nutr Biochem. 2018. PMID: 30308382
-
Rosiglitazone Regulates TLR4 and Rescues HO-1 and NRF2 Expression in Myometrial and Decidual Macrophages in Inflammation-Induced Preterm Birth.Reprod Sci. 2017 Dec;24(12):1590-1599. doi: 10.1177/1933719117697128. Epub 2017 Mar 21. Reprod Sci. 2017. PMID: 28322133 Free PMC article.
-
HYCO-3, a dual CO-releaser/Nrf2 activator, reduces tissue inflammation in mice challenged with lipopolysaccharide.Redox Biol. 2019 Jan;20:334-348. doi: 10.1016/j.redox.2018.10.020. Epub 2018 Oct 26. Redox Biol. 2019. PMID: 30391826 Free PMC article.
Cited by
-
Donor-defined mesenchymal stem cell antimicrobial potency against nontuberculous mycobacterium.Stem Cells Transl Med. 2021 Aug;10(8):1202-1216. doi: 10.1002/sctm.20-0521. Epub 2021 May 4. Stem Cells Transl Med. 2021. PMID: 33943038 Free PMC article.
-
Immunomodulatory Effect of Bifidobacterium, Lactobacillus, and Streptococcus Strains of Paraprobiotics in Lipopolysaccharide-Stimulated Inflammatory Responses in RAW-264.7 Macrophages.Curr Microbiol. 2021 Dec 14;79(1):9. doi: 10.1007/s00284-021-02708-1. Curr Microbiol. 2021. PMID: 34905100
-
Coal dust particles can upregulate the expression of NLRP3 inflammasome components in rat alveolar macrophages through phagocytosis.Sci Rep. 2025 Mar 15;15(1):8989. doi: 10.1038/s41598-025-93946-x. Sci Rep. 2025. PMID: 40089559 Free PMC article.
-
Effects of Caffeine on THP-1 Myelogenous Cell Inflammatory Gene Expression.Curr Issues Mol Biol. 2025 Apr 2;47(4):248. doi: 10.3390/cimb47040248. Curr Issues Mol Biol. 2025. PMID: 40699647 Free PMC article.
References
-
- Stoll BJ, Hansen NI, Bell EF, Shankaran S, Laptook AR, Walsh MC, et al. Neonatal outcomes of extremely preterm infants from the NICHD Neonatal Research Network. Pediatrics. 2010;126: 443–56. doi: 10.1542/peds.2009-2959 - DOI - PMC - PubMed
-
- Wilson-Costello D, Walsh MC, Langer JC, Guillet R, Laptook AR, Stoll BJ, et al. Impact of postnatal corticosteroid use on neurodevelopment at 18 to 22 months’ adjusted age: effects of dose, timing, and risk of bronchopulmonary dysplasia in extremely low birth weight infants. Pediatrics. 2009;123: e430–7. doi: 10.1542/peds.2008-1928 - DOI - PMC - PubMed
-
- Johnson TJ, Patel AL, Jegier BJ, Engstrom JL, Meier PP. Cost of morbidities in very low birth weight infants. J Pediatr. 2013;162: 243–49.e1. doi: 10.1016/j.jpeds.2012.07.013 - DOI - PMC - PubMed
-
- Keller RL, Ballard RA. Bronchopulmonary Dysplasia In: Gleason CA, Devaskar SU, editors. Avery’s Diseases of the Newborn. 9th ed Philadelphia, PA: Elsevier; 2012. pp. 658–671.
-
- Bancalari E, Walsh MC. Bronchopulmonary Dysplasia in the Neonate In: Martin RJ, Fanaroff A, Walsh MC, editors. Fanaroff and Martin’s Neonatal-Perinatal Medicine. 10th ed Philadelphia, PA: Elsevier; 2015. pp. 1157–1169.
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

