NF-κB c-Rel Is Crucial for the Regulatory T Cell Immune Checkpoint in Cancer
- PMID: 28886380
- PMCID: PMC5633372
- DOI: 10.1016/j.cell.2017.08.004
NF-κB c-Rel Is Crucial for the Regulatory T Cell Immune Checkpoint in Cancer
Abstract
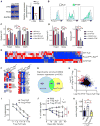
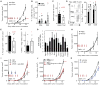
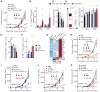
Regulatory T cells (Tregs) play a pivotal role in the inhibition of anti-tumor immune responses. Understanding the mechanisms governing Treg homeostasis may therefore be important for development of effective tumor immunotherapy. We have recently demonstrated a key role for the canonical nuclear factor κB (NF-κB) subunits, p65 and c-Rel, in Treg identity and function. In this report, we show that NF-κB c-Rel ablation specifically impairs the generation and maintenance of the activated Treg (aTreg) subset, which is known to be enriched at sites of tumors. Using mouse models, we demonstrate that melanoma growth is drastically reduced in mice lacking c-Rel, but not p65, in Tregs. Moreover, chemical inhibition of c-Rel function delayed melanoma growth by impairing aTreg-mediated immunosuppression and potentiated the effects of anti-PD-1 immunotherapy. Our studies therefore establish inhibition of NF-κB c-Rel as a viable therapeutic approach for enhancing checkpoint-targeting immunotherapy protocols.
Keywords: NF-κB; cancer; immunotherapy; regulatory T cells.
Copyright © 2017 Elsevier Inc. All rights reserved.
Figures


Comment in
-
Driving Rel-iant Tregs toward an Identity Crisis.Immunity. 2017 Sep 19;47(3):391-393. doi: 10.1016/j.immuni.2017.08.014. Immunity. 2017. PMID: 28930651
References
-
- Baumgartner JM, Gonzalez R, Lewis KD, Robinson WA, Richter DA, Palmer BE, Wilson CC, McCarter MD. Increased survival from stage IV melanoma associated with fewer regulatory T Cells. J Surg Res. 2009;154:13–20. - PubMed
-
- Beg AA, Sha WC, Bronson RT, Ghosh S, Baltimore D. Embryonic lethality and liver degeneration in mice lacking the RelA component of NF-kappa B. Nature. 1995;376:167–170. - PubMed
-
- Boissonnas A, Scholer-Dahirel A, Simon-Blancal V, Pace L, Valet F, Kissenpfennig A, Sparwasser T, Malissen B, Fetler L, Amigorena S. Foxp3+ T cells induce perforin-dependent dendritic cell death in tumor-draining lymph nodes. Immunity. 2010;32:266–278. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

