Combinatorial Signal Perception in the BMP Pathway
- PMID: 28886385
- PMCID: PMC5612783
- DOI: 10.1016/j.cell.2017.08.015
Combinatorial Signal Perception in the BMP Pathway
Abstract
The bone morphogenetic protein (BMP) signaling pathway comprises multiple ligands and receptors that interact promiscuously with one another and typically appear in combinations. This feature is often explained in terms of redundancy and regulatory flexibility, but it has remained unclear what signal-processing capabilities it provides. Here, we show that the BMP pathway processes multi-ligand inputs using a specific repertoire of computations, including ratiometric sensing, balance detection, and imbalance detection. These computations operate on the relative levels of different ligands and can arise directly from competitive receptor-ligand interactions. Furthermore, cells can select different computations to perform on the same ligand combination through expression of alternative sets of receptor variants. These results provide a direct signal-processing role for promiscuous receptor-ligand interactions and establish operational principles for quantitatively controlling cells with BMP ligands. Similar principles could apply to other promiscuous signaling pathways.
Keywords: BMP; SMAD; bone morphogenetic protein; multiplicity; promiscuity receptor-ligand interactions; signal perception; signal processing; signaling pathways.
Copyright © 2017 Elsevier Inc. All rights reserved.
Figures
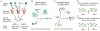
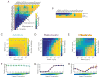




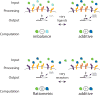
Comment in
-
Computing at the Front-End by Receptor Networks.Cell Syst. 2017 Oct 25;5(4):316-318. doi: 10.1016/j.cels.2017.10.006. Cell Syst. 2017. PMID: 29073371
References
-
- Açil Y, Ghoniem AA, Wiltfang J, Gierloff M. Optimizing the osteogenic differentiation of human mesenchymal stromal cells by the synergistic action of growth factors. J Craniomaxillofac Surg. 2014;42:2002–2009. - PubMed
-
- Afrakhte M, Morén A, Jossan S, Itoh S, Sampath K, Westermark B, Heldin CH, Heldin NE, ten Dijke P. Induction of Inhibitory Smad6 and Smad7 mRNA by TGF-β Family Members. Biochem Biophys Res Commun. 1998;249:505–511. - PubMed
-
- Atkinson DE. The energy charge of the adenylate pool as a regulatory parameter. Interaction with feedback modifiers Biochemistry. 1968;7:4030–4034. - PubMed
-
- Balemans W, Van Hul W. Extracellular regulation of BMP signaling in vertebrates: a cocktail of modulators. Dev Biol. 2002;250:231–250. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

