Protocol for the management of psychiatric patients with psychomotor agitation
- PMID: 28886752
- PMCID: PMC5591519
- DOI: 10.1186/s12888-017-1490-0
Protocol for the management of psychiatric patients with psychomotor agitation
Abstract
Background: Psychomotor agitation (PMA) is a state of motor restlessness and mental tension that requires prompt recognition, appropriate assessment and management to minimize anxiety for the patient and reduce the risk for escalation to aggression and violence. Standardized and applicable protocols and algorithms can assist healthcare providers to identify patients at risk of PMA, achieve timely diagnosis and implement minimally invasive management strategies to ensure patient and staff safety and resolution of the episode.
Methods: Spanish experts in PMA from different disciplines (psychiatrists, psychologists and nurses) convened in Barcelona for a meeting in April 2016. Based on recently issued international consensus guidelines on the standard of care for psychiatric patients with PMA, the meeting provided the opportunity to address the complexities in the assessment and management of PMA from different perspectives. The attendees worked towards producing a consensus for a unified approach to PMA according to the local standards of care and current local legislations. The draft protocol developed was reviewed and ratified by all members of the panel prior to its presentation to the Catalan Society of Psychiatry and Mental Health, the Spanish Society of Biological Psychiatry (SEPB) and the Spanish Network Centre for Research in Mental Health (CIBERSAM) for input. The final protocol and algorithms were then submitted to these organizations for endorsement.
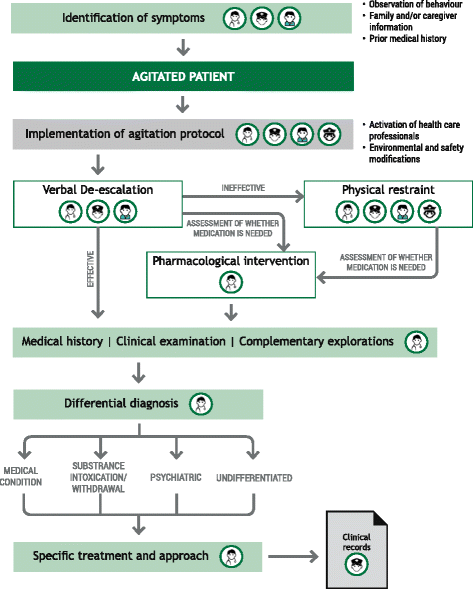
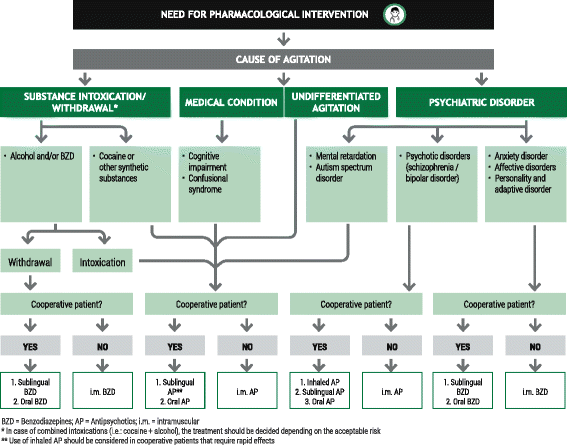
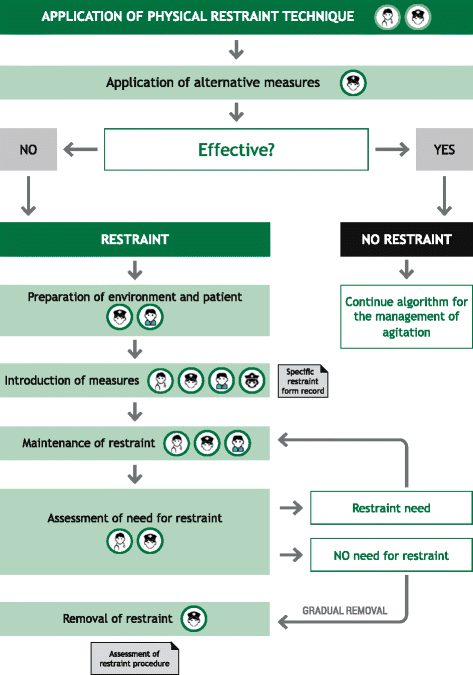
Results: The protocol presented here provides guidance on the appropriate selection and use of pharmacological agents (inhaled/oral/IM), seclusion, and physical restraint for psychiatric patients suspected of or presenting with PMA. The protocol is applicable within the Spanish healthcare system. Implementation of the protocol and the constituent algorithms described here should ensure the best standard of care of patients at risk of PMA. Episodes of PMA could be identified earlier in their clinical course and patients could be managed in the least invasive and coercive manner, ensuring their own safety and that of others around them.
Conclusion: Establishing specialized teams in agitation and providing them with continued training on the identification of agitation, patient management and therapeutic alternatives might reduce the burden of PMA for both the patient and the healthcare system.
Keywords: Inhaled loxapine; Physical restraint; Protocol; Psychomotor agitation; Verbal de-escalation.
Conflict of interest statement
Ethics approval and consent to participate
The protocol was approved by the Board of Directors from the Hospital Clínic de Barcelona.
Consent for publication
Not applicable
Competing interest
EV has received funding for research projects and/or honoraria as a consultant or speaker for the following companies and institutions: AB-Biotics, Allergan, AstraZeneca, Bial, Bristol-Myers Squibb, Dainippon Sumitomo Pharma, Elan, Eli Lilly, Farmaindustria, Ferrer, Forest Research Institute, Gedeon Richter, Glaxo-Smith-Kline, Janssen, Lundbeck, Otsuka, Pfizer, Roche, Sanofi-Aventis, Servier, Shire, Solvay, Sunovion, Takeda, Telefónica, Instituto de Salud Carlos III, Séptimo Programa Marco (ENBREC), Brain and Behaviour Foundation (NARSAD) and Stanley Medical Research Institute.
MG has received funding for research projects and/or honoraria as a consultant or speaker for the following companies and institutions: Ferrer, Janssen, Lundbeck and Instituto de Salud Carlos III.
LC has received honoraria as a consultant to the following company: Ferrer.
MB has been a consultant for, received grant/research support and honoraria from, and been on the speakers/advisory board of ABBiotics, Adamed, Almirall, Amgen, Boehringer, Eli Lilly, Ferrer, Forum Pharmaceuticals, Gedeon, Hersill, Janssen-Cilag, Lundbeck, Otsuka, Pfizer, Roche, Servier and has obtained research funding from the Spanish Ministry of Health, the Spanish Ministry of Science and Education, the Spanish Ministry of Economy and Competiveness, Centro de Investigación Biomédica en Red de Salud Mental (CIBERSAM), by the Government of Catalonia, Secretaria d’Universitats i Recerca del Departament d’Economia i Coneixement (2014SGR441), Foundation European Group for Research In Schizophrenia (EGRIS), and the 7th Framework Program of the European Union.
ML has not received funding to collaborate with any company in the health sector.
JB has received honoraria as a consultant or speaker for the following companies and institutions: Ferrer, Gilead, Janssen, Lundbeck, MSD, Otsuka, Pfizer, Servier.
RC has received funding for research projects and/or honoraria as a consultant or speaker for the following companies and institutions: Adamed, Janssen, Lundbeck and Instituto de Salud Carlos III.
MV has not competing of interest to declare.
VS has not competing of interest to declare.
NO has not competing of interest to declare.
AMA has received funding for research projects and/or honoraria as a consultant or speaker for the following companies and institutions: Otsuka, Pfizer, AstraZeneca, Bristol-Myers Siquibb, Lundbeck, Brain and Behaviour Foundation (NARSAD Independent Investigator), the Spanish Ministry of Economy and Competitiveness and Instituto de Salud Carlos III.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures
References
-
- Garrido Viñado E, Lizano-Díez I, Roset Arissó PN, et al. El coste económico de los procedimientos de contención mecánica de origen psiquiátrico en España. Psiquiatr Biológica. 2015;22:12–16. doi: 10.1016/j.psiq.2015.04.002. - DOI
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

