The Tooth: Its Structure and Properties
- PMID: 28886762
- PMCID: PMC5774624
- DOI: 10.1016/j.cden.2017.05.001
The Tooth: Its Structure and Properties
Abstract
This article provides a brief review of recent investigations concerning the structure and properties of the tooth. The last decade has brought a greater emphasis on the durability of the tooth, an improved understanding of the fatigue and fracture behavior of the principal tissues, and their importance to tooth failures. The primary contributions to tooth durability are discussed, including the process of placing a restoration, the impact of aging, and challenges posed by the oral environment. The significance of these findings to the dental community and their importance to the pursuit of lifelong oral health are highlighted.
Keywords: Aging; Dentin; Durability; Enamel; Fatigue; Fracture; Tubules.
Copyright © 2017 Elsevier Inc. All rights reserved.
Figures










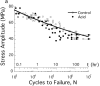

References
-
- Pashley DH. Dentin: a dynamic substrate-a review. Scanning Microsc. 1989;3(1):161–74. discussion 174–6. - PubMed
-
- Marshall GW, Jr, Marshall SJ, Kinney JH, et al. The dentin substrate: structure and properties related to bonding. J Dent. 1997;25(6):441–58. - PubMed
-
- Perdigao J, Swift EJ, Jr, Denehy GE, et al. In vitro bond strengths and SEM evaluation of dentin bonding systems to different dentin substrates. J Dent Res. 1994;73(1):44–55. - PubMed
-
- Tay FR, Pashley DH. Resin bonding to cervical sclerotic dentin: a review. J Dent. 2004;32(3):173–96. - PubMed
-
- Kinney JH, Marshall SJ, Marshall GW. The mechanical properties of human dentin: a critical review and re-evaluation of the dental literature. Crit Rev Oral Biol Med. 2003;14(1):13–29. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

