The apolipoprotein C-III (Gln38Lys) variant associated with human hypertriglyceridemia is a gain-of-function mutation
- PMID: 28887372
- PMCID: PMC5665666
- DOI: 10.1194/jlr.M077313
The apolipoprotein C-III (Gln38Lys) variant associated with human hypertriglyceridemia is a gain-of-function mutation
Abstract
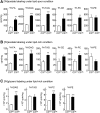
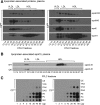
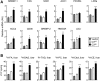
Recent cell culture and animal studies have suggested that expression of human apo C-III in the liver has a profound impact on the triacylglycerol (TAG)-rich VLDL1 production under lipid-rich conditions. The apoC-III Gln38Lys variant was identified in subjects of Mexican origin with moderate hypertriglyceridemia. We postulated that Gln38Lys (C3QK), being a gain-of-function mutation, promotes hepatic VLDL1 assembly/secretion. To test this hypothesis, we expressed C3QK in McA-RH7777 cells and apoc3-null mice to contrast its effect with WT apoC-III (C3WT). In both model systems, C3QK expression increased the secretion of VLDL1-TAG (by 230%) under lipid-rich conditions. Metabolic labeling experiments with C3QK cells showed an increase in de novo lipogenesis (DNL). Fasting plasma concentration of TAG, cholesterol, cholesteryl ester, and FA were increased in C3QK mice as compared with C3WT mice. Liver of C3QK mice also displayed an increase in DNL and expression of lipogenic genes as compared with that in C3WT mice. These results suggest that C3QK variant is a gain-of-function mutation that can stimulate VLDL1 production, through enhanced DNL.
Keywords: VLDL; de novo lipogenesis; lipogenic gene expression.
Copyright © 2017 by the American Society for Biochemistry and Molecular Biology, Inc.
Figures



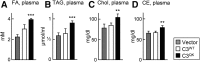
References
-
- Gangabadage C. S., Zdunek J., Tessari M., Nilsson S., Olivecrona G., and Wijmenga S. S.. 2008. Structure and dynamics of human apolipoprotein CIII. J. Biol. Chem. 283: 17416–17427. - PubMed
-
- Jong M. C., Hofker M. H., and Havekes L. M.. 1999. Role of ApoCs in lipoprotein metabolism: functional differences between ApoC1, ApoC2, and ApoC3. Arterioscler. Thromb. Vasc. Biol. 19: 472–484. - PubMed
-
- Yao Z., and Wang Y.. 2012. Apolipoprotein C–III and hepatic triglyceride-rich lipoprotein production. Curr. Opin. Lipidol. 23: 206–212. - PubMed
-
- Jørgensen A. B., Frikke-Schmidt R., Nordestgaard B. G., and Tybjaerg-Hansen A.. 2014. Loss-of-function mutations in APOC3 and risk of ischemic vascular disease. N. Engl. J. Med. 371: 32–41. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

