Evolution of protein-coupled RNA dynamics during hierarchical assembly of ribosomal complexes
- PMID: 28887451
- PMCID: PMC5591316
- DOI: 10.1038/s41467-017-00536-1
Evolution of protein-coupled RNA dynamics during hierarchical assembly of ribosomal complexes
Abstract
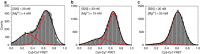
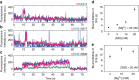
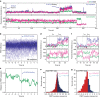
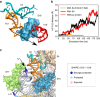
Assembly of 30S ribosomes involves the hierarchical addition of ribosomal proteins that progressively stabilize the folded 16S rRNA. Here, we use three-color single molecule FRET to show how combinations of ribosomal proteins uS4, uS17 and bS20 in the 16S 5' domain enable the recruitment of protein bS16, the next protein to join the complex. Analysis of real-time bS16 binding events shows that bS16 binds both native and non-native forms of the rRNA. The native rRNA conformation is increasingly favored after bS16 binds, explaining how bS16 drives later steps of 30S assembly. Chemical footprinting and molecular dynamics simulations show that each ribosomal protein switches the 16S conformation and dampens fluctuations at the interface between rRNA subdomains where bS16 binds. The results suggest that specific protein-induced changes in the rRNA dynamics underlie the hierarchy of 30S assembly and simplify the search for the native ribosome structure.Ribosomes assemble through the hierarchical addition of proteins to a ribosomal RNA scaffold. Here the authors use three-color single-molecule FRET to show how the dynamics of the rRNA dictate the order in which multiple proteins assemble on the 5' domain of the E. coli 16S rRNA.
Conflict of interest statement
The authors declare no competing financial interests.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

