Novel selective, potent naphthyl TRPM8 antagonists identified through a combined ligand- and structure-based virtual screening approach
- PMID: 28887460
- PMCID: PMC5591244
- DOI: 10.1038/s41598-017-11194-0
Novel selective, potent naphthyl TRPM8 antagonists identified through a combined ligand- and structure-based virtual screening approach
Erratum in
-
Publisher Correction: Novel selective, potent naphthyl TRPM8 antagonists identified through a combined ligand- and structure-based virtual screening approach.Sci Rep. 2018 Mar 6;8(1):4250. doi: 10.1038/s41598-018-21902-z. Sci Rep. 2018. PMID: 29511237 Free PMC article.
Abstract
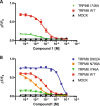
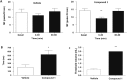
Transient receptor potential melastatin 8 (TRPM8), a nonselective cation channel, is the predominant mammalian cold temperature thermosensor and it is activated by cold temperatures and cooling compounds, such as menthol and icilin. Because of its role in cold allodynia, cold hyperalgesia and painful syndromes TRPM8 antagonists are currently being pursued as potential therapeutic agents for the treatment of pain hypersensitivity. Recently TRPM8 has been found in subsets of bladder sensory nerve fibres, providing an opportunity to understand and treat chronic hypersensitivity. However, most of the known TRPM8 inhibitors lack selectivity, and only three selective compounds have reached clinical trials to date. Here, we applied two virtual screening strategies to find new, clinics suitable, TRPM8 inhibitors. This strategy enabled us to identify naphthyl derivatives as a novel class of potent and selective TRPM8 inhibitors. Further characterization of the pharmacologic properties of the most potent compound identified, compound 1, confirmed that it is a selective, competitive antagonist inhibitor of TRPM8. Compound 1 also proved itself active in a overreactive bladder model in vivo. Thus, the novel naphthyl derivative compound identified here could be optimized for clinical treatment of pain hypersensitivity in bladder disorders but also in different other pathologies.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures
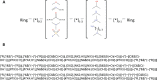
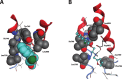
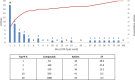
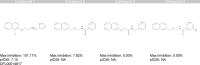


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

