Identification of Purple Acid Phosphatases in Chickpea and Potential Roles of CaPAP7 in Seed Phytate Accumulation
- PMID: 28887557
- PMCID: PMC5591292
- DOI: 10.1038/s41598-017-11490-9
Identification of Purple Acid Phosphatases in Chickpea and Potential Roles of CaPAP7 in Seed Phytate Accumulation
Abstract
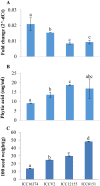
Purple acid phosphatases (PAPs) play important roles in phosphate (Pi) acquisition and utilization. These PAPs hydrolyze organic Phosphorus (P) containing compounds in rhizosphere as well as inside the plant cell. However, roles of PAPs in one of the most widely cultivated legumes, chickpea (Cicer arietnum L.), have not been unraveled so far. In the present study, we identified 25 putative PAPs in chickpea (CaPAPs) which possess functional PAP motifs and domains. Differential regulation of CaPAPs under different nutrient deficiencies revealed their roles under multiple nutrient stresses including Pi deficiency. Interestingly, most of the CaPAPs were prominently expressed in flowers and young pods indicating their roles in flower and seed development. Association mapping of SNPs underlying CaPAPs with seed traits revealed significant association of low Pi inducible CaPAP7 with seed weight and phytate content. Biochemical characterization of recombinant CaPAP7 established it to be a functional acid phosphatase with highest activity on most abundant organic-P substrate, phytate. Exogenous application of recombinant CaPAP7 enhanced biomass and Pi content of Arabidopsis seedlings supplemented with phytate as sole P source. Taken together, our results uncover the PAPs in chickpea and potential roles of CaPAP7 in seed phytate accumulation.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures






References
-
- Olczak M, Morawiecka B, Watorek W. Plant purple acid phosphatases-genes, structures and biological function. Acta Biochim. Pol. 2003;50:1245–1256. - PubMed
-
- Tran HT, Hurley BA, Plaxton WC. Feeding hungry plants: the role of purple acid phosphatases in phosphate nutrition. Plant Sci. 2010;179:14–27. doi: 10.1016/j.plantsci.2010.04.005. - DOI
-
- Duff SMG, Gautam S, Plaxton WC. The role of acid phosphatases in plant phosphorus metabolism. Physiol. Plant. 1994;90:791–800. doi: 10.1111/j.1399-3054.1994.tb02539.x. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

