Inhibition of Y1 receptor signaling improves islet transplant outcome
- PMID: 28887564
- PMCID: PMC5591241
- DOI: 10.1038/s41467-017-00624-2
Inhibition of Y1 receptor signaling improves islet transplant outcome
Abstract
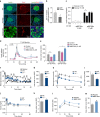
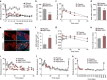
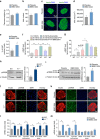
Failure to secrete sufficient quantities of insulin is a pathological feature of type-1 and type-2 diabetes, and also reduces the success of islet cell transplantation. Here we demonstrate that Y1 receptor signaling inhibits insulin release in β-cells, and show that this can be pharmacologically exploited to boost insulin secretion. Transplanting islets with Y1 receptor deficiency accelerates the normalization of hyperglycemia in chemically induced diabetic recipient mice, which can also be achieved by short-term pharmacological blockade of Y1 receptors in transplanted mouse and human islets. Furthermore, treatment of non-obese diabetic mice with a Y1 receptor antagonist delays the onset of diabetes. Mechanistically, Y1 receptor signaling inhibits the production of cAMP in islets, which via CREB mediated pathways results in the down-regulation of several key enzymes in glycolysis and ATP production. Thus, manipulating Y1 receptor signaling in β-cells offers a unique therapeutic opportunity for correcting insulin deficiency as it occurs in the pathological state of type-1 diabetes as well as during islet transplantation.Islet transplantation is considered one of the potential treatments for T1DM but limited islet survival and their impaired function pose limitations to this approach. Here Loh et al. show that the Y1 receptor is expressed in β- cells and inhibition of its signalling, both genetic and pharmacological, improves mouse and human islet function.
Conflict of interest statement
The authors declare no competing financial interests.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

