Periostin in inflammation and allergy
- PMID: 28887633
- PMCID: PMC11107676
- DOI: 10.1007/s00018-017-2648-0
Periostin in inflammation and allergy
Abstract
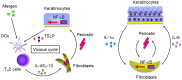
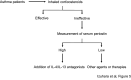
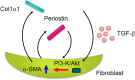
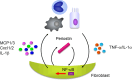
We found for the first time that IL-4 and IL-13, signature type 2 cytokines, are able to induce periostin expression. We and others have subsequently shown that periostin is highly expressed in chronic inflammatory diseases-asthma, atopic dermatitis, eosinophilc chronic sinusitis/chronic rhinosinusitis with nasal polyp, and allergic conjunctivitis-and that periostin plays important roles in the pathogenesis of these diseases. The epithelial/mesenchymal interaction via periostin is important for the onset of allergic inflammation, in which periostin derived from fibroblasts acts on epithelial cells or fibroblasts, activating their NF-κB. Moreover, the immune cell/non-immune cell interaction via periostin may be also involved. Now the significance of periostin has been expanded into other inflammatory or fibrotic diseases such as scleroderma and pulmonary fibrosis. The cross-talk of periostin with TGF-β or pro-inflammatory cytokines is important for the underlying mechanism of these diseases. Because of its pathogenic importance and broad expression, diagnostics or therapeutic drugs can be potentially developed to target periostin as a means of treating these diseases.
Keywords: Allergic conjunctivitis; Allergy; Asthma; Atopic dermatitis; Biomarker; Cross-talk; Epithelial/mesenchymal interaction; IL-13; IL-4; Matricellular protein; Periostin; Pulmonary fibrosis; Scleroderma; TGF-β.
Figures



References
-
- Ontsuka K, Kotobuki Y, Shiraishi H, Serada S, Ohta S, Tanemura A, Yang L, Fujimoto M, Arima K, Suzuki S, Murota H, Toda S, Kudo A, Conway SJ, Narisawa Y, Katayama I, Izuhara K, Naka T. Periostin, a matricellular protein, accelerates cutaneous wound repair by activating dermal fibroblasts. Exp Dermatol. 2012;21:331–336. doi: 10.1111/j.1600-0625.2012.01454.x. - DOI - PubMed
-
- Yuyama N, Davies DE, Akaiwa M, Matsui K, Hamasaki Y, Suminami Y, Yoshida NL, Maeda M, Pandit A, Lordan JL, Kamogawa Y, Arima K, Nagumo F, Sugimachi M, Berger A, Richards I, Roberds SL, Yamashita T, Kishi F, Kato H, Arai K, Ohshima K, Tadano J, Hamasaki N, Miyatake S, Sugita Y, Holgate ST, Izuhara K. Analysis of novel disease-related genes in bronchial asthma. Cytokine. 2002;19:287–296. doi: 10.1006/cyto.2002.1972. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

