An automated sampling importance resampling procedure for estimating parameter uncertainty
- PMID: 28887735
- PMCID: PMC5686280
- DOI: 10.1007/s10928-017-9542-0
An automated sampling importance resampling procedure for estimating parameter uncertainty
Abstract
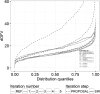
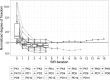
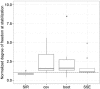
Quantifying the uncertainty around endpoints used for decision-making in drug development is essential. In nonlinear mixed-effects models (NLMEM) analysis, this uncertainty is derived from the uncertainty around model parameters. Different methods to assess parameter uncertainty exist, but scrutiny towards their adequacy is low. In a previous publication, sampling importance resampling (SIR) was proposed as a fast and assumption-light method for the estimation of parameter uncertainty. A non-iterative implementation of SIR proved adequate for a set of simple NLMEM, but the choice of SIR settings remained an issue. This issue was alleviated in the present work through the development of an automated, iterative SIR procedure. The new procedure was tested on 25 real data examples covering a wide range of pharmacokinetic and pharmacodynamic NLMEM featuring continuous and categorical endpoints, with up to 39 estimated parameters and varying data richness. SIR led to appropriate results after 3 iterations on average. SIR was also compared with the covariance matrix, bootstrap and stochastic simulations and estimations (SSE). SIR was about 10 times faster than the bootstrap. SIR led to relative standard errors similar to the covariance matrix and SSE. SIR parameter 95% confidence intervals also displayed similar asymmetry to SSE. In conclusion, the automated SIR procedure was successfully applied over a large variety of cases, and its user-friendly implementation in the PsN program enables an efficient estimation of parameter uncertainty in NLMEM.
Keywords: Asymptotic covariance matrix; Bootstrap; Confidence intervals; Nonlinear mixed-effects models; Parameter uncertainty; Sampling importance resampling.
Figures



References
-
- Lalonde RL, Kowalski KG, Hutmacher MM, Ewy W, Nichols DJ, Milligan PA, Corrigan BW, Lockwood PA, Marshall SA, Benincosa LJ, Tensfeldt TG, Parivar K, Amantea M, Glue P, Koide H, Miller R. Model-based drug development. Clin Pharmacol Ther. 2007;82(1):21–32. doi: 10.1038/sj.clpt.6100235. - DOI - PubMed
-
- Lee JY, Garnett CE, Gobburu JV, Bhattaram VA, Brar S, Earp JC, Jadhav PR, Krudys K, Lesko LJ, Li F, Liu J, Madabushi R, Marathe A, Mehrotra N, Tornoe C, Wang Y, Zhu H. Impact of pharmacometric analyses on new drug approval and labelling decisions: a review of 198 submissions between 2000 and 2008. Clin Pharmacokinet. 2011;50(10):627–635. doi: 10.2165/11593210-000000000-00000. - DOI - PubMed
-
- Marshall SF, Burghaus R, Cosson V, Cheung SYA, Chenel M, DellaPasqua O, Frey N, Hamren B, Harnisch L, Ivanow F, Kerbusch T, Lippert J, Milligan PA, Rohou S, Staab A, Steimer JL, Tornøe C, Visser SAG. Good practices in model-informed drug discovery and development (MID3): practice. CPT Pharmacomet Syst Pharmacol n/a-n/a. 2015 - PMC - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

